Fear Profiteers
How E-cigarette Panic Benefits Health Activists

EXECUTIVE SUMMARY
The rise of a vibrant market for e-cigarettes has been a blessing for many smokers. After many failed attempts to quit, e-cigarettes provide an exciting new means for smokers to reduce their exposure to the harmful aspects of combustible cigarettes or even break their life-threatening smoking habit entirely.
Although research on e-cigarettes has yet to definitively calculate the precise long-term risk associated with vaping, reputable scientific institutions are increasingly coming to the same conclusion—e-cigarettes are vastly safer than smoking, help smokers quit, and are a net positive for public health. Despite this, public perception of e-cigarette safety has declined, while demands for stricter regulation—even bans on certain e-cigarettes—have only intensified.
That the public perception of e-cigarettes diverts so radically from the actual evidence raises the question: Why? This paper makes the case that the confusion is the intended result of an orchestrated disinformation campaign led by individuals and groups that ought to be among the most supportive of lower-risk tobacco alternatives—anti-smoking health advocates.
Instead of recognizing the historic opportunity e-cigarettes represent to displace traditional smoking, powerful charities like the American Cancer Society and the Campaign for Tobacco-Free Kids, state and federal health agencies, and some academics have condemned the proliferation of vaping products. Their influence on public opinion and public policy stems largely from their image as credible, apolitical entities motivated purely by an interest in protecting public health. As their approach to e-cigarettes demonstrates, this perception is inaccurate.
In addition to their public health goals, health agencies’ and health charities’ activities are also driven by a need to defend and expand the financial resources they need to pursue their respective missions. Whereas for-profit businesses raise funds by competing for consumer dollars, non-profits—and to a certain extent, health departments—compete for charitable donations and sometimes government funding. The two basic strategies health charities and agencies employ to court funding are to: 1) raise the perceived need to address the health problem on which they focus; and 2) promote their organization’s comparative effectiveness in addressing said health problem.
This approach to fundraising is generally uncontroversial in the non-profit arena, where organizations compete intensely in an environment where both attention and charitable dollars are limited. However, when an organization is part of a government agency or endorsed by government agencies, its efforts to raise awareness of an issue and its own clout can incentivize activities that clash with sound public health policy.
Furthermore, using e-cigarettes as a case study, this paper demonstrates how this negative effect is magnified when health charities, federal health agencies, and state health departments are financially co-dependent. Though perceived as independent health charities, many of the nation’s most well-respected health non-profits are, in effect, arms of federal health agencies. Groups like the American Cancer Society (ACS) receive money from agencies, like the National Cancer Institute within the National Institutes of Health. As such, it is in ACS’ interest to support or even lobby on behalf of the National Cancer Institute as it seeks to sustain or increase the funding allocated to it in the federal budget.
In turn, health agencies like the National Cancer Institute have an incentive to boost the reputation of their supporting health charities like ACS. Not only can these charities provide strong support during budget discussions, by echoing or even executing the Institute’s efforts throughout the year, they can make the Institute appear more effective and therefore more worthy of budget allocation.
A similar process takes place at the state level, where state and local health departments “partner” with respected health charities like ACS, the American Heart Association, and the American Lung Association. The health departments divert funding to the charities, while the charities do what health departments cannot—lobby state and local governments.
These health charity-government agency alliances have proven so effective and lucrative that it has given rise to a vast, nationwide network of groups that includes government bodies at the local, state, federal and international level; charities; grassroots organizations; universities; and even scientists. While seemingly independent from one another, these entities are in fact deeply financially interwoven.
In the case of e-cigarettes, this interconnected network of health groups and advocates has helped fuel public fears about tobacco alternatives. To the general public, these disparate groups appear to have reached the same conclusion about the health effects of e-cigarettes independently of one another.
Anti-smoking activists have reason to be skeptical about nicotine products advertised as intended to “reduce harm.” But, unlike the tobacco industry’s previous efforts to promote safer cigarettes to sustain profits from smoking, e-cigarettes appear to be genuinely harm-reducing. At present, the evidence increasingly indicates that e-cigarettes not only carry significantly less risk than combustible smoking, but also help people to quit smoking and do not attract non-smoking individuals to nicotine use. In fact, smoking among both adolescents and adults is currently lower than it has ever been.
Health agencies should communicate this information in an unbiased way that allows consumers to make informed choices about the relative risk of using e-cigarettes versus smoking. Instead, health agencies, charities, health advocates, and the media have promoted the unfounded notion that e-cigarettes are as harmful as—or more harmful than—combustible cigarettes.
This campaign to restrict or ban e-cigarettes does a huge disservice to public health, decreasing the likelihood that smokers will utilize these devices as a means of quitting their deadly habit. Though concerns over e-cigarettes’ long-term effects are reasonable, that is not the impetus behind the anti-e-cigarette movement. Rather, as this paper demonstrates, it is the consequence of those groups and individuals vested with the power and funding of the government seemingly prioritizing their organizational interests over public health.
INTRODUCTION
The rise of the vaping industry has been a blessing for many smokers. After years of failed attempts to quit, e-cigarettes offer smokers a promising new approach to potentially break their life-threatening habit, while immediately reducing their exposure to the harmful elements of combustible tobacco. Since the introduction of e-cigarettes to the market, there has been a growing consensus among reputable health researchers that vaping is vastly safer than smoking. With just 1 percent of the risk,1 they have proven effective as a means of smoking cessation,2 and are likely responsible for displacing smoking among children and adults. In fact, researchers recently estimated that substituting e-cigarettes for smoking could prevent up to 7 million premature deaths in the United States.3
Given this evidence, public health advocates should welcome vaping as an effective harm reduction and smoking cessation tool. But even as the evidence and consensus on the relative safety of e-cigarettes has grown among the research community, the public’s perception of the phenomenon has grown increasingly negative, thanks in large part to the tone of news coverage. In fact, the frightening reporting on teen vaping has become observably more alarmist since January 2018.
Many recent news stories touting the risks of vaping have focused on concerns regarding one product, the Juul. The New York Times described it as the “cool-looking and sweet … vice teens can’t resist,” and blamed it for a supposed “explosion” of vaping in schools.4 Providing no better evidence than anecdotes from teachers and administrators, CNN cited the Juul as the cause of a vaping “epidemic among U.S. high schoolers.”5
National publications like Time Magazine,6 NBC News,7 The Wall Street Journal,8 USA Today,9 and countless local outlets have repeated some version of this same story, portraying the Juul as a unique threat to America’s youth, using similar and, in many cases, the same language.10
Concerns about a sudden, large increase in adolescent use of nicotine-containing products are understandable, particularly if e-cigarette use in adolescence were to lead to smoking in adulthood. However, there is no evidence, apart from anecdotes, that a significant number of adolescents are habitually vaping and zero evidence that experimental or even habitual vaping leads to use of combustible tobacco products among those who otherwise would never have used such products. If anything, the evidence shows that teen use of e-cigarettes remains a passing experimentation phase for most. The number of adolescents habitually using e-cigarettes is low. Moreover, vaping likely leads to decreased use of combustible tobacco, which is lower among teens than it has ever been, according to the latest data. That the headlines divert so jarringly from the reality of the situation and appear almost coordinated in timing, tone, and language, raises the question: Is something driving this misleading coverage?
This paper makes the case that the answer to that question is: Yes. The misleading media response is the result of an orchestrated effort meant to create confusion and public panic over electronic cigarettes—part of a strategy to pressure governments to restrict or eliminate these alternatives to traditional cigarettes. As this paper will explore, the individuals and groups behind this fear campaign are those most likely to profit from it: anti-smoking and health advocacy groups.
Put together, the facts do not indicate an epidemic of e-cigarette use among adolescents. Yet, that is exactly how some anti-smoking advocates, the news media, and even government agencies have portrayed the situation. The question this paper seeks to answer is: Why?
In its 2017 annual report on teen use of tobacco, the U.S. Centers for Disease Control and Prevention (CDC) observed that in 2016 just 11.3 percent of high school students reported using e-cigarettes at least once during the previous month.11 In the 2017 edition, released in
June 2018, the rate remained statistically unchanged, at 11.7 percent.12 While 11 to 12 percent of high schoolers reporting use of e-cigarettes might still seem like too much, the rate actually represents a 30 percent decline from the rates the CDC reported in 2015, when 16 percent of high schoolers—the most CDC ever recorded—reported e-cigarette use. Notably, the CDC survey only reports “ever use” in the previous 30 days. Habitual use, defined as using e-cigarettes on 20 or more days in the previous month, was not reported in past editions of the National Youth Tobacco Survey. However, during the zenith of e-cigarette popularity among adolescents, from 2014 to 2015, habitual e-cigarette use by teenagers was infinitesimally small, with just 2 percent of teens vaping daily and just 8 percent vaping three or more times a month. The rest can be classified as “experimental,” not habitual users.13
In November 2018, the CDC released some preliminary information gathered for the next National Youth Tobacco Survey, though not the actual data. According to these latest numbers, experimental e-cigarette use among middle schoolers, and especially high schoolers, has significantly increased since the previous survey. While in 2017, about 11.7 percent of high schoolers reported any e-cigarette use during the previous month, the newest CDC data indicate that number has increased from 20.7 percent to 20.8 percent. The survey also indicates that the proportion of high school e-cigarette users who report frequent use (20 or more days a month) has increased from 2.3 percent in 2017 to 5.7 percent in 2018.14
These early numbers have led to widespread hysteria among anti-tobacco groups and prompted FDA Commissioner Scott Gottlieb to initiate action against e-cigarette manufacturers. In September 2018, Gottlieb initiated conversations with the e-cigarette industry, asking them to take voluntary steps to address e-cigarette use by minors.15 In response, some companies, including Altria and Juul, decided to stop selling most of their flavored e-cigarettes in stores (Juul continues to sell those flavors online with enhanced age-verification procedures).16 However, in November 2018, the FDA announced its intention to ban the sale of flavored e-cigarettes, except for mint, menthol, and tobacco flavors, in all retail outlets.17
Yet, all this preliminary data indicate that habitual e-cigarette use among high schoolers, including 18-year-old adults legally purchasing these devices, is still very small at under 6 percent. Furthermore, the most recent data reveal nothing about youth use of combustible tobacco products. Since the introduction of e-cigarettes to the U.S. market, adolescent use of cigarettes has more than halved, from 15.8 percent in 2011 to 7.6 percent in 2017.18 All of this indicates that, rather than e-cigarettes acting as a “gateway” into smoking, they are more likely diverting would-be smokers into a less harmful means of consuming nicotine. Still, the number of adolescents using e-cigarettes on a daily basis remains small, at under 6 percent.
However, even these small numbers have led some public health advocates to declare all-out war on e-cigarettes, fueled by panic about the potential consequences of adolescent experimentation with nicotine, an addictive drug that may have negative effects on children’s development. But, if the number of teens habitually using e-cigarettes is small, evidence shows that the number of teens using e-cigarettes that contain nicotine is even smaller. Recent research indicates that when asked what kind of e-cigarette they used, most adolescents reported using nicotine-free varieties.19
Even as study after study demonstrates that teen vaping is not widespread, that it is declining, and that these products have the potential to save lives by displacing smoking, outlets continue to publish headlines about the “skyrocketing” number of teens succumbing to vaping and the myriad health risks supposedly associated with it.20
HOW NONPROFITS PROFIT
It might seem counterintuitive that anti-smoking advocates would spearhead a campaign to scare people away from products that reduce tobacco-related harm—a central part of their mission. But as admirable as that mission is, improving public health is not their sole objective.
All charitable and nonprofit organizations must pursue funding to support and sustain their efforts. They need income in order to pay salaries and rent and invest in the projects that advance their cause.
Like for-profit businesses that compete for customers, nonprofits must also compete with one another for limited sources of financial support, such as private donations from individuals, corporations, or foundations. Some also compete for grant money awarded by universities, governments, and other institutions.
As with for-profit ventures, competition in the nonprofit sector is fierce. In 2012 there were more than 1 million non-religious nonprofits in the United States, vying for a piece of the $190 billion donated to charities that year. That may sound like a lot of money, but allocated equally among them it amounts to less than $160,000 each—barely enough to cover the salary and operating costs for one senior employee.21 Of course, no organization or donor would be satisfied with such a low fundraising total. Thus, much of a nonprofit’s activities are aimed at convincing potential donors that their organization is more worthy of support than another.
NONPROFITS VS. GOVERNMENT-SPONSORED HEALTH ADVOCATES
Because corporate donors have for-profit motives, non-profits that receive financial support from industry often face the accusation that their activities are driven by the interests of their financial sponsors. Corporate sponsorship is not, in and of itself, sufficient evidence to question the motive of a non-profit, but it can raise other questions.
My own organization, for example, the Competitive Enterprise Institute (CEI), is a non-profit think tank that advocates for greater economic liberty. Since some of our donors are businesses—in addition to individuals and foundations—and would benefit from the adoption of such policies, our political opponents occasionally accuse of us of “shilling” on behalf of donors. But CEI makes no attempt to hide our political agenda. Our mission is to decrease regulatory hurdles, both for businesses that provide goods and services and consumers who purchase those goods and services. On the other side of the political spectrum, an organization like the Center for Science in the Public Interest (CSPI) takes the stance that consumers need to be protected from what it says are unscrupulous food companies through greater government regulation. CSPI openly advocates for policies that increase government oversight of food production, sales, and advertising. It also occasionally provides nutrition advice.
The health-focused nonprofits discussed throughout this paper are distinct from other non-profits because their advocacy is not so overt.22 Unlike overtly political non-profits, these health advocacy groups present themselves as health authorities whose advice emanates from medically accepted wisdom and whose activities are focused, without political bias, on public well-being. The air of authority these health charities seek to cultivate is heightened by the fact that they often function as an extension of government, sometimes as paid contractors of public health agencies. Not only do these health advocacy organizations receive government endorsement, they also receive financial support. Government entities are prohibited from lobbying. Instead, they route funding to health advocacy non-profits, either as direct grants or through programs sponsored by local health agencies. In this way, health advocacy nonprofits work as subcontractors for government entities, legally executing their shared political agenda. Often, a centerpiece of that agenda includes lobbying to protect or increase access to public funds.23
This, perhaps, is the most important distinction that sets public policy nonprofits like CEI or CSPI apart from the government-sponsored health policy groups discussed in this paper. Unlike traditional nonprofits to which individuals have the freedom to give money or not, the public has little choice about financially supporting government-sponsored health advocates. For the largest and most well-known of the health groups, competing for these public funds has proved far more efficient and lucrative than competing for private donations.
PURSUING FUNDING
For most non-profits, private donations are their only means of funding. But, by making the case that they provide a public service, some health advocacy groups have succeeded in securing a steady supply of taxpayer funds. As the public money for these charities has increased, so has their incentive to pursue it as a source of revenue.
This strategy has a long pedigree. “It is getting difficult relatively to get as much private money as you need,” one physician remarked at the 1910 annual meeting of the Pennsylvania Society for the Prevention of Tuberculosis—now the American Lung Association. But he noted, “if you strike for public money you can get it in greater and greater abundance. Therefore, we must turn our energies from begging money to voting money.”24 Many modern public health advocates have taken this lesson to heart.
Whether the source is private or public, nonprofit groups generally court funding using two approaches. The first is to demonstrate the need for or value of the organization’s mission. The second is to persuade potential funders of the organization’s comparative effectiveness, or “clout,” in pursuing that mission. As is often the case, the more money a charity has, the more it can do. As charities increase their activities, they also raise their clout and perceived effectiveness.
Over the last decade, the amount of money available for health-focused nonprofits, particularly from federal and state governments, has grown exponentially.25 At the same time, the number of groups willing to address those issues has also increased. Thus, while the total funding available is greater so is the difficulty in persuading people to care about and give money to any particular cause.
At the turn of the 20th century, the need to invest in combating communicable diseases was clear. Serious diseases like cholera, malaria, smallpox, polio, and measles were widespread. But, by the middle of the 20th century, public health campaigners had been so effective that most of the diseases they set out to combat were either extinct or nearly eradicated in the U.S.
The March of Dimes, for example, was established by President Franklin Roosevelt in 1938 to address polio. By 1964 there were fewer than 121 cases reported in the U.S. Rather than calling it a day, firing its employees, and returning money to donors, the March of Dimes instead shifted its focus to preventing birth defects and infant mortality, the mission we now associate with the organization. To put it in economic terms, demand for organizations addressing communicable disease declined in the mid-20th century. To survive as an entity, the March of Dimes—like any good business— wisely shifted the service it supplied to meet the changing public demand for medical research aimed at curing certain diseases.
In contrast, the missions of modern public health advocates can extend to efforts to influence public policy. For example, smoking was not considered a public health problem until the 1960s. This changed, thanks in large part to anti-smoking advocates, who promoted awareness of the dangers of smoking. As awareness of smoking-related harms increased, so did the number of groups and organizations working to address the problem.
In some cases, efforts to promote the value of a nonprofit’s mission also serve to demonstrate why it is more effective and deserving of support than another. One of the best ways to raise public awareness of a public health issue is to stoke public anxiety, which puts pressure on government entities to address the issue. A nonprofit can then point to the change in public policy to demonstrate its effectiveness.
Governments, like private donors and the public, have limited resources— time, money, and energy—to direct toward any given topic. Campaign donors or “special interests” have some sway over lawmakers’ attention. But public pressure and publicity are also effective means of enlisting government support. When there is enough public attention focused on an issue, the promise of good publicity can incentivize lawmakers to do something about it. This publicity, whether it results in policy changes or not, increases the chance that constituents will be aware of, and vote for, the lawmaker in subsequent elections.
The most effective way to generate this public attention—or at least the appearance of it—is through media coverage. As media attention increases, so does public awareness. If public attention becomes widespread enough, it can attract lawmakers’ attention. If lawmakers adopt the policy goals set forth by nonprofit advocates, it demonstrates the advocacy organization’s effectiveness. Even if lawmakers fail to institute advocates’ recommendations, they can point to such failures as evidence for the need for groups to redouble efforts and receive more donations.
Put into a formula, the lifecycle that takes this approach looks like this:
- Identify a policy goal;
- Generate media coverage to stimulate public anxiety, concern, or outrage;
- Leverage public outrage to promote policy goals;
- Leverage government/agency interest to create a feedback loop of fear;
- Fundraise based on success or failure of policies.
In many cases, organizations do not follow this formula in a linear fashion, but rather jump back and forth between the steps, often in a sort of positive feedback loop. For example, media coverage might provide the initial idea for organizations to target a specific product or behavior, or push for a specific policy goal. As public health agencies and lawmakers get involved, they generate even more media and public interest in the issue.
THE BUSINESS OF ANTI-SMOKING ADVOCACY AND GOVERNMENT FUNDING
Before the 1980s, the anti-tobacco movement was comprised primarily of passionate volunteers. They advocated for policies they believed would reduce smoking-related harms, with modest financial support. In the early days, the most prominent of these organizations, like the American Cancer Society (ACS), American Heart Association (AHA), and American Lung Association, were staffed mostly by physicians and their activities focused on research and education. Generally, they shied away from political activism and lobbying, except for one area: lobbying for federal spending on the National Institutes of Health (NIH).
The world’s largest funding source for medical research, the NIH is comprised of 21 separate institutes dedicated to specific diseases or conditions, like cancer, diabetes, kidney disease, drug abuse, aging, and many others. These individual institutes are the major funding source for many of the private charities that share their mission and often act as proxies to execute the institutes’ programs. But federal spending on each given institute is not guaranteed. Like private charities, each of the individual institutes must make its case to Congress for why it deserves a share of the federal budget. The larger the piece of the pie an institute receives, the more money it has to pass along to the private charities below it. In recent decades, this has created a significant incentive for the private charities allied with NIH institutes to lobby for budget allocations for NIH.
Throughout the 1980s, the major health charities opened government relations offices in Washington, D.C. Lobbying grew in importance as part of their activities. For example, while the American Heart Association’s 1979 annual report made little mention of lobbying, by 1984 the AHA noted that it “authored a bill that became the toughest legislation in history governing cigarette advertising,” and that “AHA’s voice will continue to be heard in Washington through the National/ Affiliate Public Affairs Network (NAPA), an effective national grassroots response system.” This network of volunteers, the report notes, “covered virtually all the powerful legislative districts in the country and almost three-quarters of all Congressional districts,” making their voices heard “whenever health issues or legislation affecting the Association, such as health issues, fundraising or research allocations came before Congress.”26
To justify its greater involvement in politics, the American Cancer Society explained in its 1987 lobbying handbook that “cancer has become political, as well has a medical, social, psychological and economic issue.” It also hints at another motivation for the organization’s involvement in politics: guaranteeing its own access to revenue. Private nonprofits, the handbook notes, are “part of an endangered species. … Therefore, we try to push government to invest more of its vast financial resources into … the cancer battle.”27
Organizational credibility was not the only reason the ACS fretted over how its increased lobbying might be viewed. It also feared the loss of its tax-exempt status. While a tax-exempt charity may use some of its funds lobbying government, this may not constitute a “substantial” part of its activities. If the limit is exceeded, the charity could lose its tax exempt status or have to pay taxes on the overage.
The Internal Revenue Service has a “safe harbor” provision that caps such spending at $1 million and requires charities to pay a 25 percent tax on funds spent lobbying over that amount.28 The ACS, with its network of affiliates around the country, was justifiably worried that its lobbying expenditures would exceed this $1 million limit. So, the ACS pursued and was granted a private ruling from the IRS that allowed it to split its nationwide network of activists into 57 divisions, divvying up the nearly $60 million it spent on lobbying so that no one “branch” spent more than $1 million on advocacy each year.29
By combining their efforts, the ACS chapters were able to raise even more public money. Today, grants for efforts aimed at preventing tobacco-related cancer take a large bite out of NIH’s $37 billion pie. While much of this money is doled out as grants to charities that use the funds to push political agendas, nonprofits tout these “wins” as securing federal funding for “research.” In 2018, for example, the American Cancer Society praised Congress after it passed a $275 million funding increase for the National Cancer Institute. “We commend lawmakers for their strong, bipartisan dedication to consistent and continual research funding reflected in this budget. Their efforts are sure to help spur groundbreaking research for years to come,” wrote the president of the Cancer Society’s Cancer Action Network, Chris Hansen. “For years these programs have struggled with flat or falling funding,” he continued, praising the budget’s $5 million increase for the CDC’s Office of Smoking and Health.30
But funding for anti-smoking groups, and hence their lobbying, is not restricted to the federal budget. Anti-tobacco advocates have also convinced state governments to hand millions of dollars over to them. For example, in 1988 Californians voted on Proposition 99, a ballot measure to triple the state’s tax on cigarettes and extract a $1.4 billion windfall from smokers over three years. Of that money, 25 percent was earmarked for tobacco control research and health education programs.31 Because the anti-smoking groups in California expected to receive some of that $350 million, the measure triggered a lobbying bonanza, with groups like the American Cancer Society, American Heart Association, and American Lung Association throwing their considerable weight and cash behind Prop 99.32
The public health charities sold their involvement in the vote as a means of convincing smokers to quit. If the cost of cigarettes rose, they argued, fewer would buy them. “The principal reason is not to raise money,” a lobbyist for the California Medical Association (CMA) stated. “If a tax were imposed and it raised nothing, we would be delighted—that would mean nobody would be buying cigarettes.”33 But the anti-tobacco groups’ efforts surrounding Prop 99 defy this claim.
For one thing, if all they wanted to do was increase the cost of smoking, they could have pursued an increased cigarette tax through the California legislature. However, at that time, the legislature had considered, but rejected, cigarette tax increases. Furthermore, going that route would have potentially limited the money from the tax hike going to the anti-tobacco groups thanks to an earlier measure approved by California voters.34
In 1978 California voters approved Proposition 13, a ballot measure that limited state spending. This law required the state to refund taxes collected that were in excess of the state’s spending limit. Though the refund would not have thwarted the health organizations’ supposed goal of increasing the cost of cigarettes (the refunds would be distributed among smokers and non-smokers alike), it did threaten the windfall the groups might receive from the tax.35
Thus, instead of a proposal in the legislature, health nonprofits pursued a referendum vote, with groups like the American Lung Association and American Heart Association devoting at least $400,000 to the “Yes on Prop 99” campaign. The ACS invested more than $200,000 in cash, loans, and staff to convince Californians to vote for Prop 99. While that may not seem like a huge amount today, especially in California, it was the largest policy advocacy project ACS had undertaken up to that point. Prop 99 won.36 In its 1990 annual report, the ACS California division claimed the tax “will help us fund health care services and education” and that “wheels are in motion to ensure that the funds are allocated and managed wisely.”37
The ACS went on to claim: “Our role at the American Cancer Society is to make sure Proposition 99 moneys are used as we intended.”38 They had good reason for concern. After the cigarette tax was approved, the health advocacy groups took to squabbling over how much money each organization should get from the revenue it would generate. In a news conference, the American Cancer Society, American Heart Association, and American Lung Association accused the California Medical Association of “playing into the hands of tobacco interests by pushing lawmakers to shift $100 million from the antismoking program to health care programs for the poor.”39 What riled the health groups was a letter sent by the CMA to legislators, in which it noted that “antismoking crusaders are not always motivated by public interest or high ideals,” and that they were “fighting for this money like jackals over a carcass.”40 Later, when California experienced a financial crisis in 1992 and attempted to shift a portion of the cigarette tax revenue to provide medical care for impoverished pregnant women, the American Lung Association successfully sued to prevent Governor Pete Wilson from diverting funds that had previously gone to it.41
Public health advocates also gain access to public funds by working as subcontractors for local health departments. For example, a 1993 CDC grant to the Florida Department of Health and Rehabilitative Services, part of its “Initiatives to Mobilize for the Prevention and Control of Tobacco Use” program, noted that the funds would be used to deploy a “Tobacco Free Florida Coalition.” The purpose of this effort, among other things, was to provide advocacy for tobacco control legislation, like increased taxes, indoor smoking bans, and restrictions on advertising and sales of tobacco.42 The funds created the role of Coalition Coordinator, a position “located at the American Cancer Society (ACS) in Tampa, Florida.”43 Of the 10 paid personnel listed for the Tobacco Free Florida, half came from either the American Heart Association, American Lung Association, or American Cancer Society. Unsurprisingly, in 2018, when the Florida legislature considered a proposal to divert some funds from the Tobacco Free Florida program to cancer research, these groups mobilized to lobby against that proposal.44
THE BUSINESS OF ANTI-SMOKING ADVOCACY AND INDUSTRY FUNDING
While the availability of government funds might have attracted the major health nonprofits to dip their toes in the world of politics, it was the deluge of private money that eventually allowed large numbers of health advocates to turn their political activism into full-time careers— and spurred a massive multiplication in the number of groups lining up to accept this philanthropy.
For pharmaceutical companies, supporting public health nonprofits proved an effective way to increase demand for their smoking cessation products. A major turning point occurred in 1991, when the U.S. Food and Drug Administration (FDA) approved the sale of the first nicotine patch available on the market, called Nicotrol. Soon after the regulatory approval of the patch, the pharmaceutical companies that manufactured and marketed the new smoking cessation products began supporting anti-tobacco health charities and activists.
Johnson & Johnson’s Robert Wood Johnson Foundation. Johnson & Johnson, the maker of Nicorette, ranks among the top 40 most profitable businesses in the world.45 One of the largest shareholders of J&J stock is its nonprofit arm, the Robert Wood Johnson Foundation (RWJF), which is correspondingly one of the world’s biggest charities and the nation’s single largest health charity.46 In any given year, RWJF donates upwards of $400 million to individuals and organizations aligned with its goals. In addition to founding and funding the Campaign for Tobacco-Free Kids (CTFK) in 1995, RWJF has since given hundreds of millions of dollars in grants to anti-smoking groups, including:
- $117 million to the Campaign for Tobacco-Free Kids;
- $150 million to the American Cancer Society;
- $99 million to the Smokeless States initiative, administered by the American Medical Association (shared with the American Cancer Society and the American Lung Association).47
These groups advocate for policy changes that would make cigarettes more expensive, more difficult to buy, and harder to use—and nicotine replacement therapies comparatively more attractive. These policies include higher cigarette taxes, restrictions on where people can smoke, increasing the minimum tobacco purchasing age to 21, restrictions on tobacco advertising, and including nicotine replacement therapies under Medicaid coverage. These regulatory shifts not only drive demand for pharmaceutical cessation products, but as the cost of smoking rises, allows the sellers of cessation products to raise prices, as well.48
Ostensibly, RWJF is a separate organization from Johnson & Johnson. Certainly there are other health issues, like obesity, for which the Foundation gives millions in charity that seem to have no bearing on J&J’s profitability. As stated earlier, the mere presence of a corporate sponsor is not sufficient to question the motive of a nonprofit. However, the two organizations work closely, as is apparent from the fact that many of the RWJF current and past board members have been long-time employees with J&J, including current board Chairman Roger S. Fine.49 Furthermore, the actions of the anti-tobacco health advocates funded by RWJF are, at the very least, strongly biased against any non-pharmaceutical nicotine. Whether this position is merely coincidental or at the direction of J&J, it appears to benefit all involved.
Stanton Glantz. RWJF also provides grants to individuals, including one of the nation’s most influential anti-tobacco advocates, Stanton Glantz. Currently a professor of medicine at the University of California, San Francisco (UCSF), Glantz entered the world of tobacco control as an activist.50 After graduating from Stanford University with a doctorate in mechanical engineering, he founded Californians for Nonsmokers’ Rights in 1981, later renamed Americans for Nonsmokers’ Rights, a pressure group that helped enact the nation’s first bans on smoking in public spaces.
Since 1996, RWJF has given more than $22 million to Americans for Nonsmokers’ Rights and its educational arm, American Nonsmokers’ Rights Foundation.51 In addition to RJWF, American Nonsmokers’ Rights received nearly $5 million between 1995 and 1999 from the California Department of Health Services—raised from California’s Prop 99 cigarette tax increase—to compile what several media outlets, including The Los Angeles Times, described as an “enemies list.” This involved monitoring and distributing information about people who spoke out against tobacco control policies at city council meetings, and even investigating a judge who had ruled unfavorably in a secondhand smoking case.52
RWJF has also given nearly $160 million to Glantz’s university since 1972, when it granted UCSF $164,000 to establish the health policy center where Glantz would later work.53 In 2002, RWJF gave UCSF a $10 million grant to establish the new Leadership Center for Smoking Cessation, headed by former RWJF President Steven Schroeder.54
Glantz has also received personal rewards, like RWJF’s Innovators in Substance Abuse Award in 2000, a $300,000 prize.55 In addition, RWJF gave Glantz $1.1 million between 2001 and 2005 to run an “educational campaign” aimed at convincing restaurant owners to support smoke-free restaurant policies by trying to convince them that they would not hurt their business.56
The Campaign for Tobacco-Free Kids. The most powerful anti-tobacco groups in the U.S. is arguably the Campaign for Tobacco-Free Kids. This organization is not only funded by RWJF, it was created by the Foundation in 1995.57 In the 1990s, its activism helped shift the debate about tobacco control from one about a personal decision affecting individual health to one about smoking’s impact on society’s most vulnerable population: children. As with most public health messaging, the focus on innocent children, who have no choice about their exposure to smoking, provided a compelling justification for the need for government regulation. Today, it remains one of the most prominent and widely cited anti-smoking and anti-vaping advocacy groups.
Apart from the Robert Wood Johnson Foundation, CTFK’s primary financial support comes from the American Cancer Society, which itself is funded with millions from RWJF and other pharmaceutical companies, like the makers of smoking cessation drugs, nicotine gums, and patches. While ACS does not publicly list its donors, some of the granting organizations that fund it disclose some grant information.58
American Cancer Society, et al. Since 1996, the American Cancer Society has received more than $20 million from the Robert Wood Johnson Foundation.59 That includes a $71,000 grant made in 2006 to ACS’ Mid- South division to lobby the Kentucky state legislature and governor’s office to include nicotine replacement therapies under Medicaid coverage.60 This was just one of many grants to various ACS divisions and other groups around the country to support expanding Medicaid coverage to include pharmaceutical smoking cessation products.61
ACS also receives around $1 million a year from lending its name and logo to smoking cessation products made by pharmaceutical companies. “The American Cancer Society views relationships with corporations as a source of revenue for cancer prevention,” Dr. Michael Thun, vice president of research at ACS, noted in a debate in 2005. “That can be construed as an inherent conflict of interest, or it can be construed as a pragmatic way to get funding to support cancer control.”62 ACS uses these funds for various projects, including lobbying for higher cigarette taxes, smoke-free space policies, and for health insurance programs like Medicaid to cover the cost of nicotine replacement therapies, including those sold by its benefactors.63
Pharmaceutical companies also fund these groups on an ad-hoc basis, supporting specific events and projects. For example, the maker of Nicorette, funds several Campaign for Tobacco-Free Kids programs, like the Global Youth Action on Tobacco and Children Helping and Motivating Parents to Stop Smoking. The latter, known by its acronym, CHAMPSS, is aimed at helping children motivate their parents to stop smoking through various means, including the use of smoking cessation products.64
There is nothing inherently wrong in pursing these funding sources, licensing their logos, or partnering with industry to promote a public health message. But it is noteworthy that ACS and Campaign for Tobacco Free Kids use many of the same tactics (particularly targeting children) for which they criticize the e-cigarette industry.
The Robert Wood Johnson Foundation, as a charitable organization, is prohibited from using its funds to lobby. However, these laws also apply to how grantees may use funds from charitable organizations. Thus, groups like CTFK, ACS, and others are instructed not to use RWJF funds for lobbying. In order to receive an RWJF grant, grantees need to demonstrate that “financial resources from other organizations, including unrestricted funds that could be used for lobbying, would be available.”65
One of the newer nonprofits to be funded by RWJF is the Public Health Law Center at the Mitchell Hamline School of Law in St. Paul, Minnesota. In 2009, RWJF began to focus on grassroots coalition building and activism.66 The following year, the Foundation announced its Health Law Initiative to provide legal expertise to public health advocates around the nation in order to “help them develop, implement, and enforce laws that help solve public health problems.” This effort, which included several law and public health schools, was to be administered by the Public Health Law Center. Since 2005, the Public Health Law Center has received around $20 million in grants from RWJF to build the anti-tobacco activist community, support public policy efforts, provide legal and technical assistance, and train future players in public health.67
Far from the days when anti-tobacco activists toiled as unpaid volunteers, todays activists are amply funded. For example, in 2016, the following organizations held in assets:
- The Truth Initiative, more than $1 billion;68
- American Cancer Society, more than $1 billion;69
- Cancer Action Network, part of the American Cancer Society, more than $4 million;70
- Campaign for Tobacco-Free Kids, more than $45 million;71
- Tobacco-Free Kids Action Fund, more than $38 million;72
- American Lung Association, more than $15 million.73
HOW ANTI-TOBACCO ADVOCATES BENEFIT BIG PHARMA
For pharmaceutical companies, it is a wise financial decision to partner with and support the efforts of anti-tobacco lobbying groups. The millions they invest in anti-tobacco campaigns is small potatoes compared to the more than $6 billion they make each year from global sales of nicotine-replacement products—and the enormous profit increases they could gain from even small changes to tobacco regulation.74
Thanks to the work of the aforementioned anti-tobacco groups, smoking has never been less inconvenient, less pleasurable, or more expensive, thanks to bans on public smoking, restrictions on cigarette advertising, and the elimination of most flavored tobacco products throughout the nation.
The positive result of these early anti-smoking efforts is that the dangers of smoking became much better and widely understood. It also helped to change the social acceptability of smoking and, correspondingly, contributed to declining smoking rates of smoking and smoking-related illness since the 1980s.75 In 2010 the CDC issued its Healthy People report, with the goal of reducing adult smoking to under 17 percent by the end of that decade.76 Not only did Americans hit that goal by 2014, but exceeded it, with smoking among adults brought down to just over 15 percent.77
However, the success of anti-smoking advocacy proved to be a threat to the survival of anti-tobacco groups. Since the 1990s, demonstrating the value of the anti-smoking groups’ mission has become much more difficult, with much of their main goal having been largely accomplished. In the developed world, few are unaware of the risks associated with tobacco use. Smoking is increasingly socially unacceptable, banned in most public places and many private places, and taxed to an extent that sustaining a smoking habit is, for many, prohibitively expensive. Partly as result, smoking is at its lowest point ever. Consequently, the threat of smoking to public health in most Western nations has lost some of its urgency.
A typical approach adopted by health campaigners in this situation has been to move the goalposts. While they started out with a goal of getting the smoking rate to below 25 percent of the population, once they hit that mark, they lowered the target goal. But, as the smoking rate dwindles, the remaining holdouts tend to be the most addicted or stubborn. As such, the regulatory approaches necessary to force them into compliance, such as bans and fines, restrict free choice so much that most Americans would find them objectionable.
Fortunately for anti-tobacco campaigners, while smoking rates in the U.S. might be approaching the point where unobtrusive nudging has little effect, declining demand for cigarettes has increased the motivation for tobacco and technology companies to develop alternative products to satisfy those who want the pleasure of smoking with fewer of the health risks. As these alternatives gained popularity, their manufacturers gave anti-tobacco activists a new target on which to focus—and thus justify their existence and expanding budgets.
THE E-CIGARETTE REVOLUTION
Invented by Chinese pharmacist Hon Lik in the early 2000s, electronic cigarettes first entered the U.S. market around 2007, but it would take until 2011 for the new product to gain its market footing. That year, just 1 percent of adults reported using e-cigarettes. By 2012, that rose to 3 percent, then in 2013 to 5 percent, and in 2014 to 7 percent.78
Because of their relative novelty and the limited information about their potential health effects, there was an understandable amount of public uncertainty and debate over what—if anything—regulators should do about the rising popularity of e-cigarettes.
By 2013, the increasing use of the new product had attracted the attention of anti-smoking advocates.79 Anti-tobacco advocacy groups began lobbying for government to regulate e-cigarettes just like traditional cigarettes, including subjecting them to tobacco taxes and banning their use in public spaces and sales to consumers under 18 years old.80 Congress had granted the agency the power to regulate tobacco products in 2009.81 Anti-smoking advocates argued that while tobacco companies were selling these new products as a safer alternative, they could still turn out to have significant harms decades later, such as more people taking up smoking.82
Promoting fear to lobby for policy change served anti-tobacco activists well when it came to publicizing and reducing smoking-related harm. They focused on two different concerns: 1) that e-cigarettes might lead to nicotine addiction and later smoking of traditional cigarettes (the “gateway” theory), and 2) the possibility that even if e-cigarette use did not lead to smoking, there could be some health risks associated with long-term vaping. While both concerns are reasonable and should be explored, the approach anti-smoking advocates pursued had the effect of spreading disinformation.
For example, in 2012 surveys found that about 13 percent of adults believed that vaping was as or more harmful to health as smoking tobacco. By 2015, more than 40 percent of adults, and 35 percent of smoking adults, held this mistaken belief.83
There is no question that e-cigarettes are less harmful than traditional cigarettes. Study after study has confirmed that they contain substantially smaller amounts of the harmful and potentially harmful chemicals found in combustible tobacco. The long-term effects are murkier, but based on what is known about the ingredients of e-cigarettes, researchers can assert with a high degree of confidence that the long-term risks are, as with the short-term, a fraction of the risks that accompany traditional smoking.
In 2015, some government public health agencies began issuing assessments of e-cigarettes’ risks that aligned with the science. That year, Public Health England, a U.K. government agency, issued an independent review of the research that found vaping to be 95 percent less harmful than smoking.84
In the U.S., in January 2018 the National Academies of Sciences, Engineering, and Medicine (NASEM) conducted its own assessment of the evidence and declared that the aerosol produced by e-cigarettes “contains fewer number and lower levels of toxicants than smoke from combustible tobacco cigarettes,” and has “apparently less risk and severity than that of combustible tobacco cigarettes.” Still, the NASEM report noted that data for the long-term effects of vaping on morbidity and mortality “are not yet clear.”85
The tentativeness of the language in the NASEM report reflects the conflicting impulses among many public health experts to accurately communicate the reduced risks associated with e-cigarettes, while not encouraging their use or promoting the idea that they are “safe.” But the fear that accurately assessing the relative risk of e-cigarettes might entice a small portion of non-smokers to pick up the habit ignores the very real possibility that such a timid endorsement (if it can be called that), buried within warnings about the unknown dangers of e-cigarettes, might discourage millions of adult smokers from switching to safer alternatives.
THE ANTI-VAPING PLAYBOOK
This fear of adolescent vaping is largely result of the efforts of anti-tobacco activists, specifically those who take an abstinence-only approach to tobacco. They believe that any tobacco product, no matter how much safer than smoking, should be viewed as harmful to the effort to reduce smoking-related harm and ought to be controlled, restricted, or banned.
Anti-tobacco activists have not been shy about their agenda, which they have been pushing long before e-cigarettes came to market. In 2005, a team of researchers led by Nigel Gray of the International Agency for Research on Cancer (IARC), an agency of the World Health Organization (WHO), detailed this plan of attack.86 They advocate, in the short term, for regulatory policies that make pharmaceutical nicotine more widely available with “reduced prices, variable size packages, and more outlets including vending machines. By contrast, tobacco availability should become progressively less easy.” In the medium term, the authors argue that policy should make non-pharmaceutical nicotine less attractive, by eliminating “attractive flavourings,” and that “non-tobacco nicotine sources need to be made more competitive with tobacco sources, with the objective that they could, over time, replace tobacco as the dominant source of the drug.” Gradually reducing the nicotine allowed in cigarettes while maintaining the higher nicotine content of pharmaceutical replacement therapies would prompt “addicted smokers who do not obtain adequate nicotine from their reduced nicotine cigarettes to supplement their nicotine intake” with pharmaceutical nicotine.87
In 2014, Eric Lindblom, a former Campaign for Tobacco-Free Kids staffer, echoed this sentiment. He argued in an academic paper that reducing non-pharmaceutical nicotine sources creates an environment where “relapse into smoking would be much more difficult (because cigarettes or cigarette-like cigars delivering adequate amounts of nicotine to support relapse would no longer be legally or readily available).”88 He further noted that, to maximize profits, the pharmaceutical industry would respond to these policies by “increasing availability of their nicotine replacement products and their related advertising and consumer education,” which Lindstrom believes will “encourage more smokers to try to quit completely and provide them with instructions on how to do so using [nicotine replacement therapies.]”
Whether they succeed in enacting these policies or not, health advocates can point to attention from media and government as evidence of the importance of their mission—and make a case to donors for why they should financially support their anti-tobacco and anti-vaping advocacy.
CASE STUDY: SNUS
This approach did not begin with e-cigarettes; it first took root years earlier with a product called snus that is popular in Sweden.89
Currently, Sweden enjoys the lowest rates of smoking of any European Union (EU) member country, with just 7 percent of adult men identifying as current smokers.90 These rates are far below even the next lowest currently in the EU—17 percent in the UK.91 The prevalence of smoking in Sweden is also much lower than in the U.S., where 15.5 percent of the adult population continues to smoke.92 Unsurprisingly, Swedish men also have the lowest rates of lung cancer in the EU. What makes Sweden different than the rest of Europe? The big difference seems to be Sweden’s embrace of snus.
Most of the harmful and potentially harmful chemicals in traditional cigarettes are produced by the process of burning paper, tobacco, and other ingredients. Thus, products that deliver nicotine without combustion are significantly less harmful. This is true not only for vaping, but for oral tobacco as well. The benefits of switching to noncombustible nicotine is not mere conjecture. There are real-world examples of large populations achieving substantial health improvements by embracing these alternative forms of nicotine. The best example is the Swedish experience with snus.
Snus, a moist tobacco powder similar to chewing tobacco, was the most popular of nicotine product in Sweden from the 19th century until the increase in popularity of cigarettes after World War II, which peaked in 1980, with about 35 of the adult population smoking. This began to change as the dangers of smoking became more widely understood.
Mortality Attributable to Tobacco, WHO 2012
Men per 100,000
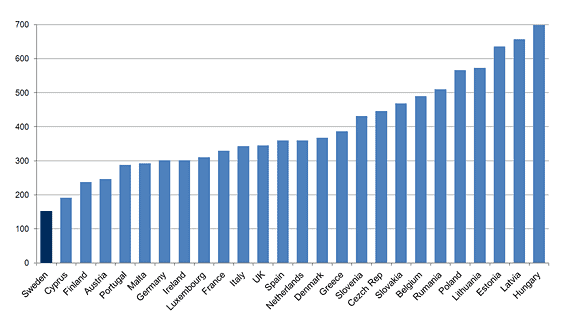
Source: World Health Organization, Mortality Attributable to Tobacco, WHO Global Report 2012, https://www.drugsandalcohol.ie/17205/.
By 1996, snus sales had surpassed cigarette sales. Today, about 15 percent of Sweden’s population uses snus.93
The benefits to public health in Sweden have been impressive. The country’s rate of tobacco-related illnesses is the lowest in Europe by far.94 Even mouth cancer, which one might expect to rise with increased oral use of tobacco, remains among the lowest in Europe.95
Unfortunately, Sweden’s success with harm-reducing tobacco alternatives was not enough to convince other countries to adopt a similar approach to harm reduction. Throughout Europe and in the U.S., anti-tobacco advocates continue to reject the possibility that products containing nicotine, made by tobacco companies,, might be able to achieve harm reduction where their public policy efforts have failed. Instead of embracing snus, the EU enacted a total ban on its sale. The public debate that led up to this ban parallels the tactics and rhetoric used by U.S. anti-tobacco activists against electronic cigarettes.
Step 1. Rely on Limited Data. While researchers generally agree that smokeless tobacco is less harmful than smoking, anti-tobacco activists have focused on a limited set of studies that suggest its use increases oral cancer risk. The assumption seems reasonable, but scientific evidence is scant, coming mostly from limited studies conducted in the U.S. on dry, powdered snuff.96
Snus, a wet tobacco product, is different than the dry kind of tobacco snuff linked to oral cancer. But the specter of potential risk provided justification enough for British anti-smoking groups to target all smokeless tobacco. Organizations like Action on Smoking and Health— an anti-smoking group created and funded by the U.K. government97— argue that restriction on sales of snus are necessary to protect children, whom they claim are being targeted by tobacco products with sweet flavors.98
In 1985, they successfully convinced Parliament to ban oral tobacco sales to anyone under 16 years old. Then four years later, anti-smoking activists persuaded the U.K. government to ban sales of all products consisting of “tobacco in fine cut, ground or particulate form which are for oral use other than smoking.”99
This was quickly followed in 1992 by a directive from the European Economic Community (later superseded by the EU), which banned sales of “new tobacco products for oral use,” among all member states, citing the oral cancer risk and the need for uniformity.100
In Sweden, this directive proved to be significant point of contention as the country considered joining the EU in 1994. The directive would have banned snus, which at the time was used by around 24 percent of the population. To secure Sweden’s accession to the Union—won in a narrow 52.3 percent to 46.8 percent vote—EU officials negotiated a deal that would allow only Sweden to opt out of the ban on smokeless tobacco, but required the product to carry a warning that said the product “causes cancer.” In 2001, the EU finally acknowledged the lack of scientific support for its ban, and replaced that warning with one that said the product “can damage your health and is addictive.”101
Since 2001, science has more thoroughly debunked the supposed oral cancer risk posed by snus. Despite this, few anti-tobacco activists have tempered their crusade against the product. Many, in fact, have doubled down, arguing that nicotine is an addictive substance and smokeless tobacco a “gateway” to smoking, a claim with no basis in research.102
Yet, some advocates recognized Sweden’s success and abandoned the abstinence-only approach. One was Clive Bates, at the time the director of Action on Smoking and Health. In 2001 he agreed with the grouping of public health professionals who asserted that less harmful tobacco products ought to be legalized and even encouraged. “The alternative proposition of ‘quit or die’, which some in our community subscribe, is wrong—totally wrong, unethical and irresponsible,” Bates wrote in 2001.103 The following year he called for an end to the snus ban.104 EU officials have considered such proposals over the years, but the anti-tobacco groups committed to an abstinence-only approach have prevented it.
As with e-cigarettes, the main supposed evidence against smokeless tobacco comes from a handful of weak and often discredited studies, produced by researchers who are themselves part of the anti-tobacco non-profit industry.
Step 2. Create Your Own Evidence. Though he began as an advocate, by the 1970s Stanton Glantz’s activism would become more effective after he moved into academia. With a doctorate in applied mechanics (his dissertation was on the mechanical interactions between the chambers of the heart), Glantz took a job at the University of California, San Francisco’s department of cardiology, where he began his career as an epidemiologist. He soon began prolifically publishing studies that supported his policy goals.105
At a 1986 meeting of anti-smoking activists, Glantz summed up his approach, advising fellow activists that “the issue should be framed in the rhetoric of the environment, toxic chemicals, and public health, rather than the rhetoric of saving smokers from themselves.”106 His aim was to focus on the harm that secondhand smoke caused to nonsmokers, particularly workers and children. Many companies were willing to fund his work.
In 1994, Glantz received a three-year grant for nearly $600,000 from the National Cancer Institute, a division of the National Institutes of Health, to study the effects of advocacy on tobacco-control policies. Part of this grant was spent on studying the effects of the tobacco industry’s campaign contributions on state tobacco regulation. This received significant attention from certain members of Congress; when lawmakers threatened to cut funding to Glantz’s project in 1996, the American Cancer Society gave Glantz a $74,000 grant to continue his project.107
In addition to his work exposing the tactics of tobacco companies, Glantz sought to combat the idea that smoke-free policies, like those pushed by his organization, Americans for Nonsmokers’ Rights, cause economic harm to the hospitality industry—an argument that proved to be a significant barrier to passing such laws in some cities. In response, Glantz produced a study that purported to show that smoke-free laws actually increase restaurants’ revenue.108 The study was then publicized by other government agencies and government-funded entities in pushing for local government ordinances to ban smoking in restaurants and bars.109
However, when Michael K. Evans, Clinical Professor of Economics at Northwestern University reviewed Glantz’s study, he noted that of the 15 cities that Glantz claimed had 100 percent smoke-free policies, only one had such ordinances, while the others allowed smoking in restaurants’ bar areas, ventilated rooms, and unenclosed patios. He also included in his data food establishments, like drive-through and fast-food restaurants, that would not likely see much effect from smoke-free ordinances.110
The cities without smoking bans that Glantz used for comparison were similarly misclassified. Evans noted that some of the cities classified as not having smoke-free policies did have restrictions on indoor smoking. After correcting for these errors, Evans found that restaurants in nine out of 12 of the cities with smoke-free laws lost revenue.111
In 1998, Glantz issued a similar study showing that bans on smoking in bars did not cause losses of revenue. This was published two months prior to the implementation of California’s bar smoking ban. Three years later, perhaps recognizing Glantz’s effectiveness, the Robert Wood Johnson Foundation chipped in another $678,819 grant to fund his “Educational campaign for restaurant owners on smoke-free restaurants.”112
Speaking before a crowd at the Seventh World Conference on Tobacco and Health in Australia in 1990, Glantz admitted that, “the main thing the science has done on the issue of [environmental tobacco smoke], in addition to help people like me pay mortgages, is it has legitimized the concerns that people have that they don’t like cigarette smoke.”113 Years earlier he had identified secondhand smoke as “the key to controlling and reducing primary smoking.”114
Step 3. Discredit Unsupportive Science—and Scientists. The passive smoker theory was anti-tobacco activists’ linchpin for a long time, but it had lost a significant amount of steam by the late 1990s, as study after study failed to demonstrate a significant link between secondhand smoke and health harms among non-smokers.
One of the these studies was a Europe-wide examination of passive smoking on the spouses and children of smokers, commissioned by the World Health Organization’s International Agency for Research on Cancer. By 1998, the report had been completed, but it remained unreleased. However, in March of that year, the U.K.’s Sunday Telegraph reported on a summary of the results in an internal WHO report that showed the study did not find a statistically significant increase in children of lung cancer risk associated with secondhand smoke.115 The British anti-smoking group Action for Smoking and Health lodged a formal complaint against the Telegraph, calling the news story “distorted and misleading,” and asked the Press Complaints Commission to investigate (the complaint was later rejected by the Commission). However, other media outlets soon published articles accusing the WHO of suppressing the study because it found its results politically unpalatable.116
The largest study on the health effects of smoking was based on data gathered from a population in California. The American Cancer Society began collecting the data in 1959. Because the data included health information from more than 35,000 non-smoking spouses, it provided the basis for the longest-running and largest secondhand smoking study ever undertaken. Funded by ACS and the Tobacco-Related Disease Research Program, an anti-smoking organization paid for by California’s cigarette tax, the analysis was undertaken by James Enstrom and Geoffrey Kabat of the University of California, Los Angeles (UCLA).
However, as with the IARC study, when the preliminary research indicated the study would fail to provide evidence of a negative passive smoking effect, both ACS and the Tobacco-Related Disease Research Program withdrew their support. Once word began to circulate about the controversial results, Enstrom and Kabat found themselves unable to find another source of foundation funding. Reluctantly, they accepted funding from the tobacco industry. Their report, published in 2003, found no significant increases in heart disease or lung cancer among non-smokers chronically exposed to secondhand smoke. Anti- smoking advocates closed ranks against Enstrom and Kabat’s paper. The ACS and Stanton Glantz mobilized their forces to undermine its findings. Glantz held a news conference in which, as he put it in an email to his followers, he would “debunk” the report. Predictably, the ACS and Glantz focused on the study having been funded by the tobacco industry, while ignoring the fact that anti-smoking groups had supported the study for 39 of its 40 years—and that it had been largely completed before the tobacco companies entered the picture.117
Enstrom and Kabat were both respected epidemiologists. Both contributed to early research that first pointed to the danger of smoking, and their integrity had never before been questioned. But the ACS and Glantz’s tactics proved effective in generating public skepticism about their findings. It is a tactic Glantz and others employ to this day. But the anti-smoking activists wanted evidence that secondhand smoke killed in order to heighten concern among nonsmokers. So, they went back to Step 2 in the anti-tobacco playbook and developed a study that would heighten concern desired result.
In 2002, residents of Helena, Montana lived under an ordinance that banned smoking in restaurants and bars. By analyzing heart attack data for the city between 1997 and 2003, including periods before, during, and after the ban, Glantz hoped to show that secondhand smoking caused heart attacks. The findings, published in BMJ—formerly the British Medical Journal—caught the attention of both health experts and journalists.118 Glantz’s study purported to show not only that secondhand smoking causes some heart attacks, but that it was responsible for most heart attacks.
At the initial presentation of the paper, the authors claimed that Helena saw a 60 percent reduction in heart attacks during the public smoking ban.119 While this estimated decline was revised down to 40 percent by the time the study was published, it still seemed impressive.120 Anti-tobacco advocates seized on the paper as proof that smoking harms even those who abstain from tobacco and as justification for more stringent tobacco control laws. They wanted to spread news of the “Helena Miracle,” as it became known in the media, across the U.S. and around the world.121 Before long, however, scientists raised concerns about the quality of the study and the validity of its conclusions.
Upon the publication of the Helena study, some researchers pointed out that the results defied the evidence. Brad Rodu, a professor of medicine at the University of Louisville wrote, that the results seemed to be merely the product of “random variation because of the small number of observations on which they are based.”122 The total number of heart attacks Glantz et al. observed was extraordinarily small, with an average of seven heart attacks per month before and after the ban, compared to four per month during the ban. When Rodu, who holds the endowed chair in tobacco harm reduction research (funded by a $3 million grant from U.S. Smokeless Tobacco Company and Swedish Match North America, Inc.),123 and his colleague Philip Cole of the University of Alabama analyzed heart attack rates in Helena beginning in 1979, they found variations in heart attacks rates similar to what Glantz’s study found during times when there was no ban on smoking in public.124
Helena was not the only city to institute this type of smoke-free policy. When researchers looked at larger cities with smoke-free policies, like San Francisco, they did not observe the change in heart attack rates found in Helena, which had a population of just over 68,000 when Glantz conducted his study. Some, like Christopher Snowdon of the Institute for Economic Affairs, have argued that Glantz and his co-authors chose Helena for this exact reason.125 With its small population, a small change in the number of heart attacks would lead to the appearance of significant changes in the rate of heart attacks. Most egregiously, Glantz’s study failed to determine whether those admitted for heart attacks to emergency rooms smoked or not. In short, it seems possible that Glantz and his fellow researchers may have cherry-picked the data to get the result they wanted.
Henry Mizgala, Professor Emeritus of Medicine at the University of British Columbia, commented when the study was published:
I am truly amazed that a study of such poor quality was not only accepted for publication in a journal with the reputation of the BMJ but was accorded widespread coverage in the lay press as having actually been published as a peer reviewed article in the print version of the journal dated April 5. This is, in my opinion, gross misrepresentation designed to provide maximal public impact in furthering the biased and unscientific opinions of these authors I have to assume that in advancing the cause of our well-meaning but scientifically challenged social engineers, correct scientific methodology can be replaced by wishful thinking.126
Echoing Mizgala, Geoffrey Kabat, at the time with the Albert Einstein College of Medicine, said, “the attempt to make claims about the effects of smoking bans based on this very weak ecologic study raises disturbing questions about our ability to distinguish between sound science and wishful thinking.”127
Step 4. Use the Media. Criticisms of Glantz’s Helena study did little to dampen the media’s enthusiasm for the results, with The New York Times calling it the “Secondhand Smoking Gun.”128 The U.K.’s Independent claimed that the study showed that smoking bans “could halve number of heart attacks.”129 The study was even cited by the CDC when it issued a warning about smoking in 2004 and the Surgeon General’s report in 2006, which concluded that exposure to secondhand smoke “has immediate adverse effects on the cardiovascular system and causes coronary heart disease and lung cancer.”130
In contrast, the Enstrom and Kabat article was controversial and, as such, should have generated significant media attention—exactly what Glantz and others feared. But that is not what happened. In fact, the study was hardly mentioned in the media at all. Only 60 newspapers worldwide, and only 15 in the U.S., covered the study, with most high- circulation papers ignoring it altogether. Those that did mention the study portrayed it in a negative context. One story in The Sacramento Bee stated: “A new UCLA study downplaying the effects of secondhand smoke on the health of smokers’ spouses is being condemned even before it has appeared in print.” Instead of focusing on the results, the story highlighted crticisms of the study, even citing Stanton Glantz, who said that “as a piece of science, it’s pretty crappy.”131
Two sociologists who investigated the media’s curious response to the study, Sheldon Ungar of the University of Toronto and Dennis Bray of Germany’s Helmholtz-Zentrum Geesthacht, found that this “self-silencing” occurred because it defied the “regime of truth” about secondhand smoke created by anti-smoking activists like Glantz. “The media perceive NO controversy and hold that the jury is IN,” they concluded.132 [Capitals in original] When the media want to report on tobacco harms, it is often Glantz—and often Glantz alone—to whom they turn.
Glantz’s true gift is in creating studies that capture the attention of journalists and headline writers, an approach he has used successfully against products beyond traditional cigarettes. While he admitted that smokeless tobacco was less harmful than smoking, Glantz dismissed the idea that such products could play any role in tobacco harm reduction. “It’s like saying you can reduce your harm if you jump out of a fifth-story window instead of a 20th story window,” he said in 2009.133 He portrayed snus and other less harmful alternatives as merely a ploy by tobacco companies to bolster their business, tweeting in 2013 that “big tobacco promoted snus in Europe to keep people smoking … not for harm reduction.”134
Glantz has used this argument, more recently, against electronic cigarettes, claiming they are a “gateway” product aimed at youngsters that, rather than help people quit, helps smokers circumvent the smoke-free laws he helped enact, discourages quitting,135 creates “dual users,” and “expand[s] the overall nicotine use market.”136 “We’ve made huge progress in denormalizing tobacco use and making the cool thing to do to be a nonsmoker,” Glantz recently told National Public Radio. “Until e-cigarettes came along, total tobacco and nicotine consumption was dropping, and at least with youth it’s now increasing.”137
In reality, much of the data indicate that Glantz and his allies are dangerously wrong about electronic cigarettes. Contrary to Glantz’s claim that tobacco use among adolescents is increasing, it is lower than it has ever been. Traditional smoking is the lowest ever recorded and teen vaping is also declining since its peak, as CDC data show.138 None of the empirical studies purporting to show the “gateway effect”— that adolescent e-cigarette leads to smoking—have done so.139 And there is a growing body of evidence that indicates that vaping reduces the use of traditional cigarettes among youth. If e-cigs have any kind of “gateway” effect, it is as a gateway to quit smoking.140
Dr. Jonathan Winickoff, another outspoken anti-vaping advocate and professor at Harvard Medical School, recently likened e-cigarettes to “bioterrorism” and called Juul “a massive public-health disaster.”141 Winickoff, like Glantz, has received funding from the Robert Wood Johnson Foundation. He has also received grants from the Truth Initiative, for research that purported to show evidence for the debunked idea of “thirdhand smoking” and linking vaping to “popcorn lung,” a deadly respiratory disease caused by diacetyl exposure.142 There is not a shred of evidence linking smoking—which has hundreds of times more diacetyl than any vape—to the condition.143 He also has advocated banning smoking in public housing and evicting tenants if they violate the policy.144
In 2016, Glantz and colleagues released a paper asserting that smokers who use e-cigarettes reduce their chances of quitting by 28 percent, but the results were rigged from the start. They reached this conclusion by pulling data from 40 other studies of e-cigarettes, but only included those studies with participants who were current smokers and had already tried e-cigarettes. Thus, the only people the study looked at were those smokers for whom e-cigarettes had already proved an unsuccessful means of smoking cessation.145
Researchers like Peter Hajek, professor of clinical psychology and director of the Tobacco Dependence Research Unit at Queen Mary University in London, pointed out the flaw in this study, calling it “grossly misleading.” He continued:
Imagine you recruit people who absolutely cannot play piano. There will be some among them who had one piano lesson in the past. People who acquired any skills at all are not in the sample, only those that were hopeless at it are included. You compare musical ability in those who did and those who did not take a lesson, find a difference, and report that taking piano lessons harms your musical ability. The reason for your finding is that all those whose skills improved due to the lessons are not in the sample, but it would not necessarily be obvious to readers.146
Some of the authors whose studies were used in Glantz’s meta-analysis also criticized the study, saying it misrepresented their work. Ann McNeill, professor of tobacco addiction at the National Addiction Centre at King’s College London, said:
This review is not scientific. The information included about two studies that I co-authored is either inaccurate or misleading. In addition, the authors have not included all previous studies they could have done in their meta-analysis.” I believe the findings should therefore be dismissed. I am concerned at the huge damage this publication may have—many more smokers may continue smoking and die if they take from this piece of work that all evidence suggests e-cigarettes do not help you quit smoking; that is not the case.147
Even the Truth Initiative, set up with funds resulting from the largest settlement against tobacco companies and no proponent of e-cigarette use, lambasted Glantz’s study as “invalid” and “scientifically inappropriate.”148 But, once again, the news media primarily focused on the study’s finding that e-cigarette users are half as likely to give up smoking as those who do not vape, as Reuters reported.149
More recently, some anti-smoking activists have tried to convince the public that e-cigarettes are not a safer alternative to smoking and are actually more dangerous than cigarettes. For instance, Glantz recently told reporters that “there’s growing evidence that in terms of adverse effects on the lung, e-cigarettes are actually worse than conventional cigarettes.”150 In reality, a growing body of evidence points to the relative safety of e-cigarettes and other non-burning tobacco alternatives, as well as the health benefits for smokers who switch.151
Another major tactic anti-smoking activists often use to rebut research showing any potential benefit from non-traditional forms of tobacco and nicotine is to allege that the research is funded by, or the researchers connected to, the tobacco industry, but, ironically, many of the same activists, nonprofits, and researchers have their own ties to industry—including the tobacco industry. Still, science should be judged on its own merits, not on the researchers’ affiliations.
THE ANTI-TOBACCO-BIG PHARMA ALLIANCE
Michael Siegel, a professor of community health sciences at Boston University and a former student of Glantz, has documented some anti-tobacco researchers’ financial ties to big pharma.152 Siegel believes these financial ties might explain their “strong bias” when it comes to e-cigarettes and their commitment to the idea that the only safe alternative to smoking are those products provided by pharmaceutical companies.
For example, when R.J. Reynolds introduced dissolvable tobacco lozenges, Glaxo lobbied the FDA to ban the product. “These products are being marketed not as substitutes, but to be used in addition to cigarettes,” company representatives wrote in a letter to the agency.153 Glantz and the Campaign for Tobacco-Free Kids spoke out in support of the move, with the latter arguing that “these new smokeless products and the marketing used to promote them appeal to kids and pose a public health threat.”154
THE ANTI-TOBACCO-BIG TOBACCO ALLIANCE
Big pharma is not the only industry invested in hampering harm-reducing tobacco alternatives. Activists have another powerful, albeit surprising, benefactor to their efforts: big tobacco. While it might seem strange that major tobacco companies would lend support to anti- smoking advocates who ostensibly want to eliminate their business, these sorts of strange-bedfellow alliances are common in politics and have a uniquely powerful influence on regulation in the U.S., particularly with regard to tobacco.
Coalitions of “Bootleggers and Baptists,” a term coined by economist Bruce Yandle, are a phenomenon that occurs when groups with contrary ultimate ends work toward common policy objectives.155 One group provides a moral justification for the policy while the other stands to gain economically. This alliance of morality with financial profit cansignificantly affect the course of regulation.156 The best relevant example of this occurred in 1998 when the attorneys general of 46 states, an assortment of trial lawyers, and the four largest tobacco companies agreed to the multi-state Tobacco Master Settlement Agreement (MSA).
The accord sought to settle a series of lawsuits filed by the states against tobacco manufacturers for the medical care expenses that tobacco products supposedly cost state health agencies. The agreement imposed advertising and sales restrictions and required the tobacco companies to pay the states $10 billion up front and payments of around $15 billion a year thereafter. In return, the companies were granted immunity from future lawsuits by the states.
For large tobacco companies, government, and health advocates, the MSA seemed like a win-win-win deal. The large tobacco companies— Philip Morris, R. J. Reynolds, Brown & Williamson, and Lorillard— signed on to it because it ended the threat of state lawsuits. Furthermore, though they would have to raise their prices to cover the state payments, the MSA protected established tobacco companies from price competition by small, off-brand cigarette makers that did not join the agreement. In fact, the price increases instituted by the major tobacco companies were higher than was necessary to cover MSA payments.157
Furthermore, because the proportion of payments was determined based on each company’s market share, the deal eliminated any incentive for the big companies to compete with one another on the basis of price. The deal also stipulated that for every percentage of market share the major companies lost to non-participating competitors, they could reduce their payments to states in turn, unless those states enacted statutes that prevented price competition among non-participating manufacturers— which all those states party to the MSA had done. By eliminating price competition and adding new restrictions on marketing, the companies believed the MSA would protect them from competition indefinitely.158
State and local governments were the biggest winners in the deal, receiving hundreds of billions of dollars throughout the next two decades, on top of the revenue they collected on cigarette sales.
For anti-tobacco activists, the deal meant new restrictions on the industry and more money for anti-tobacco activism. The deal set aside $1 billion to found the American Legacy Foundation, renamed the Truth Initiative in 2015, a nonprofit dedicated to eliminating tobacco use among young adults.159 Activists hoped states would grant much of the annual payments to anti-smoking initiatives. However, the deal did not require states to spend the money in any particular way, so most of it went to fill budget gaps and toward other projects, with states spending less than two cents of every MSA dollar they received on tobacco harm reduction.160
There were other consequences that tobacco companies perhaps did not foresee. The sharply rising cost of maintaining a smoking habit, combined with increasing awareness of its risks, likely led nicotine consumers to begin demanding a satisfying and safer alternative to smoking. In other words, the MSA may have helped drive consumer demand for electronic cigarettes.
This growing market for alternative tobacco products created new competitors for traditional tobacco companies and manufacturers of pharmaceutical nicotine. Declining cigarette sales, and declining cigarette tax revenues, also threaten to tighten the spigot of money flowing to anti-tobacco activists. With their financial futures intertwined, these two disparate groups found their interests aligned, facing a common foe.
THE ANTI-TOBACCO-PUBLIC HEALTH ALLIANCE
Years before the e-cigarette boom, in 2002 Philip Morris lobbied Congress to grant the FDA authority to regulate tobacco products and to approve or reject new products. Smaller competitors, like R.J. Reynolds, British American Tobacco, and Lorillard, opposed FDA regulation, arguing that the new rules would essentially protect Philip Morris’ dominant position.161 Anti-smoking advocates not only sided with Philip Morris, but partnered with the company to draft legislation.162
By 2009, that legislation took the form of the Family Smoking Prevention and Tobacco Control Act (TCA), which Congress enacted that year. It gave the FDA authority to regulate cigarettes and other tobacco products, banned flavored cigarettes (except menthol), required packaging to carry more explicit warnings, and mandated that any product put on the market after 2007 must obtain pre-market approval from the agency.163 Though some tobacco companies criticized aspects of the law, it was endorsed to one degree or another by anti-tobacco groups, tobacco companies, and pharmaceutical firms, including the Campaign for Tobacco-Free Kids, American Heart Association, American Lung Association, American Cancer Society, Altria Group (Philip Morris’ parent company), R.J. Reynolds, and GlaxoSmithKline.164
The TCA’s requirement of pre-market approval for new products led some, like anti-tobacco lawmaker Sen. Mike Enzi (R-Wyo.), to dub the bill the “Marlboro Protection Act.”165 FDA approval would necessarily be a long and expensive process, which created a major hurdle for new products and smaller companies, protecting existing products and entrenched players. While the law originally only granted the FDA authority over traditional tobacco, like cigarettes, roll-your-own tobacco, and smokeless tobacco, it allowed the agency to “deem” new types of products as falling into the “tobacco product” category.
Since the TCA’s enactment, the major cigarette companies have sought to meet the changing demands of consumers by entering the burgeoning e-cigarette market, including R.J. Reynolds with its Vuse product and Altria Goup (Philip Morris) with its MarkTen brand. Yet, the legacy tobacco companies combined maintain less than a 40 percent share of the vapor market. The rest is comprised of international brands and small startup firms, like Pax Labs (maker of the Juul), which compete fiercely for consumers with an ever-expanding array of products.
It did not take long for anti-smoking activists to turn their attention toward this rising e-cigarette market and launch campaigns to convince the FDA to deem these new products as “tobacco products,” thereby subjecting them to the same rules and requirements placed on cigarettes and other traditional tobacco products by the TCA.
At the time, the biggest product on the e-cigarette market was the Vuse. Introduced by R.J. Reynolds in 2013, the Vuse is defined as a “cigalike,” an e-cigarette designed to look like traditional cigarette. It comes in two varieties that either are entirely disposable or come with pre-filled disposable cartridges. But the Vuse was not the only e-cigarette on the market, and it faced increasing competition from other cigalikes and premium vapor products—e-cigarettes that bear little resemblance to traditional cigarettes and allow users to refill cartridges with e-cigarette “juice.” Many consumers are attracted to these types of e-cigarettes because the variety is virtually unlimited, allowing users to choose or even create whatever flavor they prefer at whatever strength of nicotine they want, or with no nicotine at all.
Against this backdrop of increasing competition, some of the major tobacco companies, like Altria, began to push for the FDA to extend its regulatory authority to e-cigarettes. Though R.J. Reynolds had previously opposed FDA regulation of tobacco products, it endorsed this so-called “deeming” of e-cigarettes as tobacco products.166 This move would require all products on the market after 2007, including Reynolds’ own Vuse, to obtain FDA approval prior to being allowed on the market, a requirement that, according to the FDA’s own estimates, would eliminate 99 percent of the vaping market.167
While a large company like Reynolds could easily absorb the increased costs, its smaller competitors—which made up the bulk of the premium vapor product segment—would be crushed by the additional expenses. However, that is not the reason R.J. Reynolds gave for supporting imposing new regulations on its own industry. Instead, the company appealed to the same arguments long used by anti-tobacco advocates. In a 119-page document submitted to the FDA, the tobacco company argued that the agency should regulate all nicotine-containing products to protect consumers’ health. While the company argued that e-cigarettes, due to their lower risk profile, ought to be regulated differently than traditional tobacco, it called for the agency to ban flavored e-cigarettes and e-cigarette juice, noting that, “FDA should not allow such products to be sold or marketed,” because, as R.J. Reynolds spokesman David Howard asserted, “open-system vapor products create unique public health risks.”168 That argument mirrored those advanced by anti-tobacco groups, which claimed that the variety of flavors offered by open systems and e-cigarette “juices” has “fueled the popularity of e-cigarettes … among youth.”169
In 2013, the CDC’s annual survey on tobacco use among adolescents found that e-cigarette use had increased among teenagers between 2011 and 2012—not entirely surprising, considering it had only been on the U.S. market for a few years.170 Mitch Zeller, director of the FDA’s Center for Tobacco Products, pointed to the study’s finding as evidence of the need for government regulation. However, Zeller may not have been a disinterested observer. Zeller was a veteran of the war on smoking, working at the FDA under then-Commissioner David A. Kessler, the architect of the government’s eventual regulation of the tobacco industry.171 After leaving the FDA in 2000, Zeller became an executive with the American Legacy Foundation, later renamed the Truth Initiative. He also later worked as a political consultant on behalf of pharmaceutical companies, until he was appointed Director of the FDA’s Center for Tobacco Products in 2013.172
News media coverage of the CDC report noted only that it found increasing e-cigarette use among teens, without asking if the increase in risk-reducing products might be responsible for the observed declines in traditional tobacco. Activists also picked up on the study, with Matthew Myers, president of the Campaign for Tobacco-Free Kids, asserting that the study “indicates that e-cigarettes could be a gateway to nicotine addiction and use of other tobacco products,” even though it found nothing of the sort.173 In fact, the study found that cigarette and overall tobacco use among high schoolers and middle schoolers had decreased.174
Activist-researchers then added fuel to the fire with studies that seemed to substantiate the purported risks that e-cigarettes posed to children and justify government intervention. This came in the form of the 2014 meta-analysis by Glantz and colleagues, in which they argued that without strict regulation of these new products it was “unlikely that they will contribute to reducing the harm of tobacco use and could increase harm by perpetuating the life of conventional cigarettes.” They based this claim on the unsubstantiated assumptions that e-cigarettes re-normalize smoking, delay smokers from quitting, and attract children to smoking.175 While Glantz’s language in the study was fairly measured, he was far less objective speaking to the media, at one point describing e-cigarettes as “cigarettes on training wheels.”176
Much of this coverage focused on the dangers of adolescents trying e-cigarettes and getting hooked on nicotine, and possibly progressing from e-cigarettes to real cigarettes. For example, a 2015 study published in the Journal of the American Medical Association found that non-smoking teenagers who reported using e-cigarettes were more likely to subsequently use traditional cigarettes. The authors—led by Adam Leventhal and David Strong of the University of Southern California and Matthew Kirkpatrick of the University of California-San Diego School of Medicine—claimed that this indicated that e-cigarette use was associated with increased risk of combustible tobacco use among adolescents.177
The study’s authors were careful to note that this was not evidence of a “gateway” effect, and that their study merely showed an association, not that e-cigarettes caused subsequent smoking in teens. It is possible, in fact, highly likely, that whatever underlying factor might predispose teenagers to try e-cigarettes would also predispose them to smoke.
However, the news media ignored that caveat, instead implying that e-cigarette experimentation among teens was responsible for their initiating smoking later on. CTFK’s Myers told NBC News that the study “provides troubling new evidence that use of electronic cigarettes by youth who had not previously smoked could lead to use of cigarettes and other smoked tobacco products.”178
The rhetoric and media coverage also ignore the fact that even among the small percentage of teens who have ever vaped or vape regularly, the majority of teens vape nicotine-free varieties, according to data collected before the Juul’s rise in popularity. “Nicotine is considered harmful to the developing teenage brain,” University of Michigan professor Richard Miech told the Associated Press in 2016, but according to his research, most teens who vape are not getting any nicotine at all.179 This “raises big questions about how U.S. health officials are portraying the threat of e-cigarettes to youths,” he said, noting that according to his research only about 22 percent of 12th-grade vapers and 13 percent of eighth-grade vapers reported vaping with nicotine.180 While it is possible that more teens are vaping the Juul, and since Juul does not offer a nicotine-free version, that more teens are vaping nicotine, but none of the scientific data confirm this.
Still, that has done little to cool anti-tobacco activists’ zeal to brand e-cigarette use among teens as an epidemic. Instead, they argue that government’s failure to regulate e-cigarettes has “created a nationwide human experiment we could pay the price for over decades,” as Myers put it in 2015.181 In May 2016, the activists got their wish, as the FDA’s Center for Tobacco Products, led by Mitch Zeller, officially deemed e-cigarettes tobacco products to be subject to the Tobacco Control Act’s rules and restrictions.
Since the FDA’s deeming rule, tobacco giants like R.J. Reynolds and Altria, along with the vaping industry, have supported measures to amend the Tobacco Control Act, allowing products on the market prior to the FDA’s deeming rule in 2016 to be grandfathered in and exempted from its requirements for pre-market approval. But, thanks in large part to lobbying by anti-tobacco activists, like the Campaign for Tobacco-Free Kids, such measures have failed to gain approval to date.182
As a result, when the deeming rule’s requirements go into effect in 2022, it will all but guarantee that few, if any, e-cigarettes will remain on the market. This is because, unlike traditional cigarettes, no e-cigarettes were on the market prior to 2007—the original predicate date written into the TCA. Thus, unlike traditional cigarettes, all e-cigarettes will be required to exit the market until they obtain pre-market FDA approval—a lengthy and expensive process that few companies will be able to afford, with no guarantee of eventual approval of any new products.183 Since 2016, the FDA has received 367 premarket tobacco applications. As of October 2018, none have been approved.184
Although the deeming rule did nothing to increase the safety or decrease the attractiveness of traditional cigarettes, anti-tobacco advocates still hailed the FDA’s action as a win for public health.185 Meanwhile, the regulatory hurdles make it virtually impossible for companies to introduce lower risk alternatives, and thus protect traditional tobacco from would-be competitors by eliminating the industry’s incentive to develop safer products.
Still, activists and groups bent on destroying the possibility of safer nicotine alternatives were not satisfied. “This is a critical first step, but it’s only a first step,” Myers told the medical news services Stat News in May 2016.186 By the end of 2017, the anti-smoking activism industry had found a new target: the Juul.
ACTIVISTS VS. JUUL
As we have seen, the anti-smoking coalition uses a tried and tested formula to create “demand” for the the anti-smoking groups’ “service” as public health advocates. First, they generate moral public panic over a health threat, real or imagined. They do this using media and allied researchers to raise awareness and concern among the general public. Anti-smoking activists stoke public anxiety to create public demand for government policies favorable to their mission and the perpetuation of their organizations. At the moment, this strategy is on full display in the campaign against the Juul.
As noted, a small percentage of teens use e-cigarettes. According to the latest CDC report on the topic, published in June 2017, about 1.7 million high schoolers reported using e-cigarettes during the previous month. However, it found that only 1.1 percent (about 23,000 individuals) used e-cigarettes on a daily basis.187 Since 2015, youth vaping rates have been on the decline. Among those who do vape—at least according to previous research—most do not vape nicotine.188 Yet in recent months, news outlets have published hundreds of stories about the “explosion” of vaping among high school students, blaming the Juul for this nonexistent trend.
A major driving force behind the media’s focus on the Juul seems to be the Campaign for Tobacco-Free Kids. For years, CTFK has relentlessly promoted the notion that there has been a massive uptick in youth vaping, supposedly caused by tobacco companies “luring kids with candy-flavored e-cigarettes … putting a new generation of kids at risk of nicotine addiction.”189 Before this organization honed in on the Juul, it had targeted other e-cigarette products, like the Blu e-cigarette, for using marketing tactics “right out of big tobacco’s playbook.”190 In 2017, when the Juul surpassed sales of its competitors, it became CTFK’s primary target.191
Unfortunately for reporters, apart from the vapor companies themselves, one of the few sources for information for a given device is the anti- tobacco organizations. For instance, a January 2018 CTFK news release contained some notable phrasing regarding the Juul, such as the idea that it is particularly attractive to teens because of its “sleek and discreet design,” which “looks quite similar to a USB flash drive … [and] can be charged in the USB port of a computer,” and enticing flavors like “cool mint, crème brulee, [sic] and fruit medley.” The release also noted that the Juul pods, according to the Juul website, contain “5% nicotine … the equivalent amount of nicotine as a pack of cigarettes, or 200 puffs.”192 The following month, The Truth Initiative, released a Juul “fact sheet,” with nearly identical language.193
In the wake of these releases, news outlets carried stories about the Juul “epidemic” among teenagers, with similar—and in some cases identical language—to the CTFK release. For example, in a February 5, 2018, story, Buzzfeed News described the Juul as “the sleek, trendy, USB- shaped e-cigarette” that contains nicotine in amounts “approximately equivalent to one pack of cigarettes, or 200 puffs,” and has “gained somewhat of a cult following among young adults.” The Buzzfeed story asserted that Juul’s “yummy flavors, and discreet design … makes it a hit with teens.”194
On April 3, 2018, an AOL News story described the Juul as “spreading in popularity like a wildfire.” The Juul was particularly concerning, according to the story, because it “resemble[s] a flash drive, can easily be hidden among school supplies or concealed in pockets and backpacks. It is USB chargeable, thereby appealing to tech-savvy millennials.” This, the article contends, is “the next generation of high-tech smoking,” in which “e-cigarettes now resemble sleek flash-drives and are so discreet that kids can actually vape right in class.” AOL News also noted that “each pod is the nicotine equivalent of 200 puffs, or an entire pack of traditional cigarettes,” and that it “comes in a variety of kid-friendly flavors—including mango, cool cucumber, fruit medley, and crème brulee.[sic]”195
A March 15, 2018 CNN headline asked if teen use of the Juul was the “Health problem of the decade?”196 Originally written for California Healthline, the story also described the Juul as “a small, sleek device [students] can easily conceal in their palms” that “resembles a flash drive.” Each Juul pod, the CNN story continues, “delivers about 200 puffs, about as much nicotine as a pack of cigarettes.” Like many others, this story also claimed that the Juul was “appealing to youth because it can be easily charged on a laptop … and the pods are available in flavors such as mango, mint and crème brûlée.”
When asked about it, the author of the CNN story (originally published by Kaiser Health News), Ann B. Ibarra, explained that she found “very limited resources with information about the Juul.” Sources on which she had to rely included documents produced by the Campaign for Tobacco-Free Kids, the Truth Initiative, and the manufacturer’s website, among others.197
One particular claim from the Campaign for Tobacco-Free Kids has gotten considerable play in the news media. According to CTFK, the 2017 edition of the CDC’s annual National Youth Tobacco Survey found 11.3 percent of high schoolers and 4.3 percent of middle schoolers reported vaping in the past month.198 Many news outlets cited this figure in stories about e-cigarette use among teens. For example, an April 6, 2018 CNN story noted that a “sharp spike in vaping and the use of e-cigarettes by students has grabbed the attention of the U.S. Food and Drug Administration 1.7 million high school students said they had used e-cigarettes in the previous 30 days.”199 While the numbers are technically correct, the CDC did not find a rise in vaping among teenagers. In fact, the study noted a massive 30 percent decline in e-cigarette use among high schoolers.200
Journalists are not entirely to blame for such confusion. As noted, there are few sources of information on vaping aside from anti-tobacco activist groups. To make matters worse, government and academic researchers tend to oversell the threat posed by vaping while down- playing or dismissing any evidence that contradicts their message or might mollify public anxiety. In this case, even if reporters did read the CDC report, they still might have missed its finding about declines in teen vaping, because the report minimized that fact.
For example, while the report’s summary notes the sharp decline in both tobacco use and vaping by 2016, it states that these one-year declines were “offset by increases in hookah and e-cigarette use [since 2011], resulting in no significant change in any tobacco use.” It adds, “In 2016, e-cigarettes remained the most commonly used tobacco product among [students].”201 The news media mostly followed suit, underscoring the CDC’s negative takeaway about the trends in teen e-cigarette use since 2011 and ignoring the declines in overall tobacco use observed that year.
The sudden focus on the Juul is not surprising to anyone familiar with the vaping industry. On the one hand, its status as the most popular vaping technology currently on the market makes it a visible target for advocates of heavy-handed vapor regulations. On the other hand, there is nothing unique about the Juul compared to other e-cigarettes. While activists and news stories highlight the fact that it looks like a USB device and can be charged in a laptop’s USB port, they ignore—or are not aware— that many e-cigarettes are also small and sleek and almost all can be charged via USB port.202 The Juul’s flavor options are similar to those offered for other e-cigarettes and its nicotine content, 5 percent by volume, is roughly equivalent to that of the Vuse, with the Vuse’s Vibe model having 59 milligrams of nicotine by milliliter and about 4.8 percent nicotine by volume.
The one aspect of the Juul that does seem novel is its apparent ability to provide a satisfying “throat hit,” the sensation of nicotine hitting the throat. According to ex-smokers, this sensation is often missing when they switch to other e-cigarettes or other forms of nicotine replacement. The Juul reportedly produces a throat hit similar to cigarettes, which might account for its rising popularity among ex-smokers and could make them less likely to relapse back into smoking.203
Most importantly, there is no scientific evidence to support the idea that the Juul—which has only been on the market since 2015 and is already the most vaping popular product on the market among adults and teens—has caused an increase in teen vaping, as opposed to simply displacing other brands. Yet, media interest in the Juul has significantly increased in the past year. Analysis with Google Trends, which tracks search term popularity over time, shows that news coverage of the Juul use since the product’s introduction was relatively flat until the end of 2017, when it sharply increased.
Since the beginning of 2018, there have been a few noticeable spikes in interest in the product, beginning in late January and continuing throughout May.
These spikes seem to follow the release of documents from anti-tobacco activists, like the Campaign for Tobacco-Free Kids, as well as action taken by local health agencies, such as the Washington State Health Department, which, on March 23, 2018, warned that the Juul was increasingly popular “especially among youth.”204 Three weeks later, on April 12, the Delaware Department of Health and Social Services issued a news release advising parents and teachers about “the recent trend among youth known as “JUULing.”205
Spikes also follow letters sent by members of Congress, like one sent by Rep. Frank Pallone (D-N.J.) to the FDA on March 9, 2018, asking the agency to apply the Tobacco Control Act to e-cigarettes,206 and a May 6, 2018, letter from Sen. Chuck Schumer (D-N.Y.) to the FDA, calling on the agency to ban “kid-friendly flavors.”207
It is not clear if these efforts were coordinated, but there is evidence that CTFK disseminated much of the information on which many state departments of health relied for their own messaging. Emails obtained via a state freedom of information law request show that on December 19, 2017, CTFK Director of Research Laura Bach sent an email to “State Program Contacts,” including the program manager for the Delaware Department of Health and Social Services’ Tobacco Prevention and Control Program. In the email, Bach noted the “increasing popularity among youth of JUUL devices, an e-cigarette that can be concealed easily because it looks like a USB flash drive and has cartridges that are available in many kid-friendly flavors,” and that “JUUL now has the highest market share (in tracked channels), surpassing Reynolds’ Vuse as the most popular e-cigarette product.”208 [Capitals in original] To support these assertions, Bach, in her email, cited three news stories on the popularity of the Juul among students. Then, when the Delaware Department of Health and agencies in other states issued their own warnings, it spurred even more media coverage.
This is a good example of how anti-tobacco activists create a positive feedback loop of fear. They use media coverage to stoke public panic, then responsive government bodies issue their own statements, which seem to validate the original calls for concern, stimulating even more news coverage and more concern.
Coordinated efforts targeting the Juul appear to have begun in earnest around February 2018, when the Campaign for Tobacco-Free Kids and the Public Health Law Center—which is funded by the Robert Wood Johnson Foundation and coordinates RWJF’s grassroots initiative—began planning a Juul-focused webinar scheduled for April 12. A February 15 email from Law Center Senior Staff Attorney Susan Weisman to Jeffrey Willett of the Truth Initiative, Erika Mansur of the Arizona Attorney General’s Office, and members of the California Department of Public Health (CDPH) requested their participation in the webinar. Weisman hoped that CDPH would discuss the “dangers for youth and young adults associated with e-cigarettes” and “industry influence, infiltration into schools and using a former superintendent/principal as a mouthpiece.”209
Communications surrounding planning for the webinar indicate that it was a coordinated collaboration by the Public Health Law Center and CTFK, with input from several individuals within CDC (such as Margaret Mahoney, formerly with the Public Health Law Center), the California Department of Public Health, the Massachusetts Department of Public Health, and Delaware Health and Social Services.
Following the April 12 webinar, these health bodies issued their own warnings about the Juul, further stimulating media interest, in a way that was well-timed to coordinate with other anti-tobacco policy efforts.
Rep. Pallone’s March 9 letter to the FDA cited the recent flurry of media stories on the Juul as reason for the agency to reconsider delaying its deeming regulations on e-cigarettes to 2022, which, he argued, would leave children at risk. On the same day, the Campaign for
Tobacco-Free Kids launched a lobbying effort to include e-cigarettes in state and local smoke-free laws that ban smoking in public places, like bars, restaurants, and workspaces.210 Days later, a coalition of anti- tobacco groups, including CTFK, sued the FDA, alleging that its delay of the deeming rule violated federal statutes.211
Throughout March, both federal and state departments of education and health issued warnings about the Juul, stimulating even more media coverage.212 For example, in April the CDC released a poster titled, “Parents and Teachers: That USB Stick Might be an E-cigarette,” which specifically targeted Juul use.213
On April 18, the Campaign for Tobacco-Free Kids, Truth Initiative, American Cancer Society, and other groups sent a letter to the FDA, demanding that the agency take steps against the Juul, including “immediately ordering the removal of any Juul flavors.”214 They justified their request by claiming that “extensive media reports and educators have documented the skyrocketing popularity of Juul among middle and high school students across the U.S., as well as on college campuses.” The same day, Rep. Dick Durbin (D-Ill.) and 10 other lawmakers sent a similar letter to the FDA, also citing news reports as evidence and asking the agency to “remove kid-friendly e-cigarette … flavorings from the market.”215
In September 2018, the FDA launched a nationwide effort to crack down on the sales of Juul to minors.216 “We commend the FDA and Commissioner Scott Gottlieb for recognizing the seriousness of the problem and taking enforcement action to prevent Juul sales to youth,” Campaign for Tobacco-Free Kids president Matthew Myers wrote in a statement. “However, the FDA must do more by taking off the market Juul flavors like mango and cool cucumber that clearly appeal to children and adolescents, preventing the introduction of look-alike products and subjecting e-cigarettes to FDA review of their public health impact, as required by law.”217
The sudden interest in the Juul overlaps not only with policy efforts aimed at the federal government, but also with action at the state level. For instance, on June 5, 2018, San Francisco voters went to the polls to decide on Proposition E, a measure to ban the sale of flavored tobacco products, including menthol cigarettes and all non-tobacco flavors of e-cigarettes.218 Groups funding the “yes” campaign included the Campaign for Tobacco-Free Kids, American Cancer Society, American Heart Association, and American Lung Association.219 The single largest funder of the “vote yes” campaign is former New York Mayor Michael Bloomberg, with a donation of $1.3 million (Bloomberg also gave CTFK nearly $10 million in 2016).220
Many news stories on funding around the Prop E vote pointed out that the opposition, mainly tobacco and vaping companies, spent much more money than the “yes” effort, with R.J. Reynolds donating around $9 million to the “vote no” campaign (perhaps because Prop E banned menthol cigarettes), but the financial math does not do justice to the influence of the activists, both in and outside of government. For example, on April 24, 2018, the California Department of Public Health—which has given millions to groups like American Nonsmokers Rights to monitor “enemies” of tobacco control—launched a media campaign called “Flavors Hook Kids,” aimed at combating “the tobacco industry’s latest strategies aimed at getting young people hooked on nicotine.”221 To get their message out, CDPH awarded San Francisco advertising firm Duncan Channon, Inc. a $325 million five- year grant to run slick television ads, which included young actors paid to vape on camera, beginning in January 2018.222 While CDPH is not technically lobbying, since none of its ads specifically referred to Prop E, it is hard to believe that the media blitz did not influence voters who ultimately approved the proposition.223
The Juul panic has also been used to support demands by anti-smoking activists that the FDA suspend online sales of e-cigarettes.224 Notably, this change would not harm either cigarette makers or the largest marketers of e-cigarettes. Instead, it would primarily harm small retailers of e-cigarettes who, unlike the larger tobacco companies, rely almost entirely on Internet sales.225
The FDA has made several major concessions to the anti-smoking/health advocacy lobby in recent years. In August 2017, FDA Commissioner Scott Gottlieb, a physician and former vaping company board member, announced an educational campaign to warn adolescents not about the dangers of smoking, but about the harms of e-cigarettes.226 Regardless of how well-intentioned the new FDA teen-focused campaign may be, its emphasis on only the possible harms of vaping will likely help perpetuate the inaccurate idea that vaping is as dangerous as smoking. While it might succeed in getting fewer teens to try electronic cigarettes, it will also likely scare smokers—adult and adolescent—away from using e-cigarettes as a means of reducing or quitting the much deadlier habit of smoking. More recently, the FDA introduced plans to reduce the nicotine content allowed in tobacco products. There is little evidence that this will lead either to more smokers quitting or cause smokers to smoke more to get the same nicotine fix. But it would bring us closer to what may be the true end-goal of anti-tobacco advocacy—a scenario where consumers’ only legal choice for full-strength nicotine is provided by pharmaceutical companies as smoking cessation products.
CONCLUSION
The scientific evidence is clear that vaping, while probably not harmless, is far less harmful than smoking, helps smokers quit cigarettes, and has not attracted non-smoking teens to smoke. A large body of evidence indicates that e-cigarette use among adolescents has never been significant and is currently decreasing. But anti-tobacco activists and the news media continue to promote the false notion that we are in the midst of a teen vaping epidemic.
Public fears about the supposed dangers of e-cigarettes appear to be fueled by anti-smoking advocates’ successful execution of a long-term and well-funded strategy to promote fears about e-cigarettes, then exploit those fears to lobby for progressively stricter regulations on— and the eventual elimination of competitors to—nicotine replacement products.
The loser in this scenario is the public, especially smokers. As the evidence indicates, increasing e-cigarette taxes, eliminating flavors, and restricting entry into the vaping market will have little to no benefit for adolescents, but potentially catastrophic consequences for adult smokers. We are already beginning to see the effects of this sort of regulatory approach in nations that embrace e-cigarettes as a safer alternative seeing rates of smoking decline while neighboring countries with stricter rules have seen smoking rates remain high.227
By scaring consumers about the unknown risks of vaping, generating panic that e-cigarettes attract children, and using government to strip away any advantage non-combustible nicotine products may have over cigarettes, anti-smoking advocates are making it much more difficult for smokers to quit. As a result, millions of people around the world who might have switched to less harmful alternatives, like e-cigarettes or snus, instead will continue to smoke and continue to die. Some have been surprisingly frank about their willingness to sacrifice adult smokers to protect adolescents from the unknown risks posed by e-cigarettes. In a September 2018 statement, FDA Commissioner Scott Gottlieb admitted that his agency “may have to narrow the off ramp for adults, to close the on ramp for kids.”228
Anti-smoking advocates often portray big tobacco companies as “merchants of doubt” that pour large sums money into efforts to obfuscate the evidence that their products are harmful to health, in order to keep making money. But the strategies employed by anti-tobacco activists to push for anti-vaping policies are not that different: using hyperbolic language in the media and misleading research to spread the patently false idea that vaping is as bad as or even worse than smoking.
There is nothing wrong with health groups accepting grants from industry, even from companies with a financial stake in their mission, with their advocating for regulatory changes they believe will benefit public health, or with utilizing the media to raise the profile of their issues or organizational clout. However, there is something wrong with health- focused groups using taxpayer funds to obscure facts, lobby government, collude with activists in government agencies, and create unwarranted public panic.
For public health regulation to do more good than harm, regulators need to base decisions on an objective analysis of sound research and a thorough examination of the potential unintended consequences of policy proposals. They should not make decisions based on assumptions, blind fear, or political pressure.
NOTES
- William E Stephens, “Comparing the cancer potencies of emissions from vapourised nicotine products including e-cigarettes with those of tobacco smoke,” Tobacco Control, Vol. 27 (2018) pp. 10-17, http://tobaccocontrol.bmj.com/content/27/1/10.info.
- Daniel P. Giovenco and Cristine D. Delnevo “Prevalence of population smoking cessation by electronic cigarette use status in a national sample of recent smokers,” Addictive Behaviors, Vol. 76 (2018), https://www.researchwithrutgers.com/en/publications/prevalence-of- population-smoking-cessation-by-electronic-cigarett.
Emma Beard, Robert West, Susan Michie, and Jamie Brown, “Association between electronic cigarette use and changes in quit attempts, success of quit attempts, use of smoking cessation pharmacotherapy, and use of stop smoking services in England: time series analysis of population trends,” BMJ, Vol. 354, No. i4645 (2016), https://www.bmj.com/content/354/bmj.i4645.
- David T. Levy, Ron Borland, Eric N Lindblom, et al. “Potential deaths averted in USA by replacing cigarettes with e-cigarettes” Tobacco Control, Vol. 27, No. 1 (2018), http://tobaccocontrol.bmj.com/content/early/2017/08/30/tobaccocontrol-2017- 053759.
- Ginia Bellafante, “Cool-Looking and Sweet, Juul Is a Vice Teens Can’t Resist,” New York Times, February 16, 2018, https://www.nytimes.com/2018/02/16/nyregion/juul-teenagers-vaping- ecigarettes-dangers.html.
Kate Zernike, “‘I Can’t Stop’: Schools Struggle With Vaping Explosion” New York Times, April 2, 2018, https://www.nytimes.com/2018/04/02/health/vaping-ecigarettes-addiction- teen.html.
- Roni Selig, Davide Cannaviccio, and Charlotte Hawks, “Vaping now an epidemic among US high schoolers,” CNN, April 6, 2018, https://www.cnn.com/2018/04/06/health/high-schools-vaping-epidemic/index.html.
- Jamie Ducharme, “Teens Are ‘Juuling’ at School. Here’s What That Means,”
Time, March 27, 2018, http://time.com/5211536/what-is-juuling.
- Anna B. Ibarra, “Why ‘juuling’ has become a nightmare for school administrators,” NBC News, March 26, 2018, https://www.nbcnews.com/health/kids-health/ why-juuling-has-become-nightmare-school-administrators-n860106.
- Anne Marie Chaker, “Schools and Parents Fight a Juul E-Cigarette Epidemic,”
Wall Street Journal, April 4, 2018, https://www.wsj.com/articles/schools-parents-fight-a-juul-e-cigarette- epidemic-1522677246.
- Josh Haffner, “Juuling is popular with teens, but doctor sees a ‘good chance’ that it leads to smoking,” USA Today, October 31, 2017, https://www.usatoday.com/story/money/nation-now/2017/10/31/juul-e-cigs- controversial-vaping-device-popular-school-campuses/818325001.
Editorial Board, “Warning: Vaping teens becoming a new generation of nico- tine addicts,” USA Today, April 8, 2018, https://www.usatoday.com/story/opinion/2018/04/08/vaping-teens-becoming- new-generation-nicotine-addicts-editorials-debates/489748002/.
- Michaela Feldmann, “Vaping: A growing epidemic among high school and middle school students,” KSFY, April 9, 2018, http://www.ksfy.com/content/news/Vaping-A-growing-epidemic-among-high- school-and-middle-school-students-479210133.html.
Editorial Board, “Teens who vape: Will a new craze invite a health crisis?” Chicago Tribune, April 9, 2018, http://www.chicagotribune.com/news/opinion/editorials/ct-edit-vaping- nicotine-schools-health-20180403-story.html.
Andrew F. Johnston, “Vaping Now an Epidemic Among US high Schoolers,” KMIR, April 9, 2018,
http://kmir.com/2018/04/09/vaping-now-an-epidemic-among-us-high-schoolers. Nicolette Perrone, “Officials: vaping craze turning into a crisis among youth,” News Channel 10, April 9, 2018, http://www.newschannel10.com/story/37916180/officials-vaping-craze- turning-into-a-crisis-among-youth.
- Ahmed Jamal, Andrea Gentzke, S. Sean Hu, Karen A. Cullen, Benjamin J. Apelberg, David M. Homa, and Brian A. King, “Tobacco Use Among Middle and High School Students—United States, 2011–2016,” Morbidity and Mortality Weekly Report, Centers for Disease Control and Prevention, June 16, 2017, https://www.cdc.gov/mmwr/volumes/66/wr/mm6623a1.htm#F1_down.
- Teresa W. Wang, Andrea Gentzke, Saida Sharapova, et al., “Tobacco Product Use Among Middle and High School Students—United States, 2011–2017,” Morbidity and Mortality Weekly Report, Centers for Disease Control and Prevention, June 8, 2018, https://www.cdc.gov/mmwr/volumes/67/wr/mm6722a3.htm.
- Thomas A. Wills, Rebecca Knight, Rebecca J. Williams, Ian Pagano, and James
D. Sargent, “Risk Factors for Exclusive E-Cigarette Use and Dual E-Cigarette Use and Tobacco Use in Adolescents,” Pediatrics, Vol. 135, No. 1, pp. e43-e51, http://pediatrics.aappublications.org/content/early/2014/12/09/peds.2014-0760.
- U.S Centers for Disease Control and Prevention, “Notes from the Field: Use of Electronic Cigarettes and Any Tobacco Product Among Middle and High School Students—United States, 2011–2018,” November 16, 2018, https://www.cdc.gov/mmwr/volumes/67/wr/mm6745a5.htm?s_cid=mm6745a5_w.
- U.S. Food and Drug Administration, “Statement from FDA Commissioner Scott Gottlieb, M.D., on new steps to address epidemic of youth e-cigarette use,” news release, September 12, 2018, https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ ucm620185.htm.
- Nathan Bomey, “Marlboro maker Altria halts sales of flavored vaping liquid as FDA weighs ban, USA Today, October 25, 2018, https://www.usatoday.com/story/money/2018/10/25/altria-group-flavored-vaping- liquid/1759794002/.
Sheila Kaplan and Jan Hoffman, “Juul Suspends Selling Most E-Cigarette Flavors in Stores,” New York Times, November 13, 2018, https://www.nytimes.com/2018/11/13/health/juul-ecigarettes-vaping- teenagers.html.
- Chris Kirkham, “FDA to ban flavored e-cigarettes at U.S. convenience stores,” Reuters, November 15, 2018,
https://www.reuters.com/article/us-usa-vaping-flavors/fda-to-ban-flavored-e- cigarettes-at-us-convenience-stores-idUSKCN1NK26T.
- U.S Centers for Disease Control and Prevention, “Notes from the Field: Use of Electronic Cigarettes and Any Tobacco Product Among Middle and High School Students—United States, 2011–2018.”
- Richard Miech, Megan E. Patrick, Patrick M. O’Malley, and Lloyd D. Johnston, “What Are Kids Vaping? Results from a National Survey of U.S. Adolescents,” Tobacco Control, Vol. 26, Issue 4 (2017), pp. 386–391, http://tobaccocontrol.bmj.com/content/26/4/386.
- Joshua Yan, “Why e-cigarette use is skyrocketing among the youth,”
Los Angeles Times, September 7, 2017, https://bit.ly/2sHHphu.
- Elizabeth T. Boris, “The Nonprofit Sector in the United States: Size and Scope,” PowerPoint presentation, Urban Institute, Center on Nonprofits and Philanthropy, February 2, 2013, https://pdfs.semanticscholar.org/presentation/47af/24c3b53ba5554a7bf4086b4 d9f06b4ac01de.pdf.
- While the word “charity” is used to describe public health non-profits throughout this paper, their tax designation as 501(c)3 is the same as other non-profits.
- The pursuit of public funding for a nonprofit organization is not limited to health charities, but that is the focus of this paper. Examples of how health charities cultivate a perception that their mission is wholly aimed at public health, as opposed to politically minded, can be seen in the way the organization describes itself: “The American Cancer Society saves lives by helping people stay well, helping people get well, by finding cures and fighting back.” American Cancer Society, “American Cancer Society Creates a World with More Birthdays at MoreBirthdays.com, officialbirthdayblog.com and on Facebook,” accessed, October 29, 2018, http://pressroom.cancer.org/releases?item=152. Such descriptions are often repeated by official government websites, like the Florida Public Health Department, which notes: “The American Cancer Society saves lives by helping you stay well by preventing cancer or detecting it early, helping you get well by being there for you before and after a diagnosis, by finding cures through groundbreaking discovery and fighting back through public policy.” Tobacco-Free Florida, “Relevant Links,” accessed, October 29, 2018, http://tobaccofreeflorida.com/relevant-links.
ACS is also frequently cited as a contributor to or source for national governmental health policies and recommendations. For example, see National Cancer Institute, “Common Cancer Types,” accessed, October 29, 2018, https://www.cancer.gov/types/common-cancers and http://bit.ly/2OUIZVS.
- Transactions of the Sixth Annual Meeting of the National Association for the study and prevention of Tuberculosis (1910), p. 93, https://babel.hathitrust.org/cgi/pt?id=hvd.hneeyt;view=1up;seq=9.
- Kyle Kinner and Cindy Pellegrini, “Expenditures for Public Health: Assessing Historical and Prospective Trends,” American Journal of Public Health, Vol. 99, No. 10, (October 2009), pp. 1780–1791, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2741526.
- James T Bennett and Thomas DiLorenzo, CancerScam: Diversion of Federal Cancer Funds to Politics (New Brunswick, NJ: Transaction Publishers, 1997), p. 24.
27 Ibid., p. 25.
28 U.S. Internal Revenue Service, “LTR 9145039: Charitable Organization Divisions Are not Affiliated,” statement 19, Tax Analysts, November 19, 1991, pp. 4374-4377.
29 Ibid., p. 29.
- Cancer Action Network, “FY 18 Budget Prioritizes Medical Research and Cancer Prevention,” news release. March 22, 2018, https://www.acscan.org/releases/fy-18-budget-prioritizes-medical-research- and-cancer-prevention.
- Thomas E. Novotny and Michael B. Siegel, “California’s Tobacco Control Saga,” Health Affairs, Vol. 15, No. 1, pp. 58-72. (1996), http://bit.ly/2u7xlhI.
- Richard C. Paddock, “Health Care Groups Join to Push Cigarette Taxes,”
Los Angeles Times, February 2, 1987,
http://articles.latimes.com/1987-02-02/news/mn-169_1_tobacco-institute.
- Ibid.
- Edith D. Balbach, Michael P. Traynor, Stanton A. Glantz, “The Implementation of California’s Tobacco Tax Initiative: The Critical Role of Outsider Strategies in Protecting Proposition 99,” Journal of Health Politics, Policy and Law, Vol. 25, No. 4, pp. 689-715 (August 2000), https://www.ncbi.nlm.nih.gov/pubmed/10979517.
- T Bennett and DiLorenzo, CancerScam, p. 65.
- Daniel Carson, “Campaign Laws Clouded by Taxpayer Subsisidies for Prop 99 Cigarette Tax initiative,” San Diego Union, October 17, 1988.
- American Cancer Society, California Division, 1990 Annual Report, p. 1.
- James T Bennett and Thomas DiLorenzo, CancerScam, p. 68. 39 Ibid., p. 67.
- Ibid.
- Daniel M. Weintraub, “Heat Put on Wilson Over Anti-Smoking Funds,”
Los Angeles Times, February 21, 1992, https://lat.ms/2JoZ47S.
- James T. Bennett and Thomas J. DiLorenzo, “From Pathology to Politics: Public Health in America,” (Abingdon, UK: Routledge, 2017) p. 67. This author was unable to locate a copy of the original application for the CDC grant from either CDC or the Florida Department of Health.
43 Ibid., p. 66.
- Jim Rosica, “Advocates rally to save Tobacco Free Florida funding,” Florida Politics, March 14, 2018, http://floridapolitics.com/archives/258942-protect-tobacco-free-florida.
- Fortune 500, Johnson & Johnson, accessed June 20, 2018, http://fortune.com/fortune500/johnson-johnson.
- Foundation Center, “Fiscal Totals of the 50 Largest Foundations in the U.S. by Total Assets, 2014,” accessed June 20, 2018, http://data.foundationcenter.org/#/foundations/all/nationwide/top:assets/list/2014.
- Christopher Snowdon, “The Anti-Smoking Movement,” Velvet Glove, Iron Fist, October 17, 2010,
https://velvetgloveironfist.blogspot.com/2010/10/anti-smoking-movement.html. Karen K. Gerlach and Michelle A. Larkin, “The Smokeless States Program,” Chapter Two in The Robert Wood Johnson Foundation Anthology: To Improve Health and Health Care, Volume VIII, https://www.rwjf.org/en/library/research/2005/01/to-improve-health-and- health-care-volume-viii.html.
- Norm Kjono, “Tobacco tax initiative is a costly pro-business hoax,” Los Angeles Daily Journal, November 2, 2006. http://www.forces.org/writers/kjono/pdf/la_daily.pdf.
- Robert Wood Johnson Foundation, Leadership and Staff, “Roger S. Fine,” accessed October 16, 2018,
https://www.rwjf.org/en/about-rwjf/leadership-staff/rwjf-board/roger-s-fine.html.
- Glantz holds the position of Professor of Medicine at the University of California, San Francisco, but he is not a medical doctor. While his post-doctoral work in cardiology at Stanford focused on computer modeling of heart functions, his Ph.D. is in mechanical engineering. Truth Tobacco Industry Documents, Stanton
A. Glantz Cirriculum Vitae, prepared May 20, 1991, accessed June 10, 2018, https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=xyph0111.
- Robert Wood Johnson Foundation, Grants Explorer, accessed June 21, 2018, https://rwjf.ws/2KqEhg8.
- Terri Hardy, “Smokers spy tax: using tax funds for ‘enemies list’ not what public intended, critics say,” Los Angeles Daily News, December 6, 1999, http://bit.ly/2tDGcHF.
- Robert Wood Johnson Foundation Grants Explorer, accessed June 21, 2018, https://rwjf.ws/2ITdRH4.
- James Bornemeier, “Taking on Tobacco: The Robert Wood Johnson Foundation’s Assault on Smoking,” Chapter 1 in The Robert Wood Johnson Foundation Anthology: To Improve Health and Health Care, Volume VII, p. 5, http://bit.ly/2tCSNKZ.
- Innovators Awards program, Innovator Award Recipients in 2000, accessed June 10, 2018, http://innovatorsawards.org/innovators.
- Robert Wood Johnson Foundation, Grants Explorer Grants 44070 and 52810, accessed June 21, 2018, https://rwjf.ws/2Km9rWG.
- Robert Wood Johnson Foundation, “The Campaign for Tobacco Free Kids,” March 26, 2009,
https://www.rwjf.org/en/library/research/2009/03/the-campaign-for-tobacco- free-kids.html.
- ACS has received funding from: Pfizer, maker of the smoking cessation drug Chantix; Novartis, maker of over-the-counter nicotine gums; and AstraZeneca, maker of smoking cessation drugs. Pfizer, “Pfizer and The Pfizer Foundation Award Multi-Year Grants to Support Cancer and Tobacco Control around the World,” news release, February 19, 2008,
http://press.pfizer.com/press-release/pfizer-and-pfizer-foundation-award-multi- year-grants-support-cancer-and-tobacco-contro.
American Cancer Society, “Corporate Impact Awards Recognize Companies’
$1 Million-Plus Annual Gifts to American Cancer Society,” news release, November 19, 2009, http://pressroom.cancer.org/releases?item=204.
- Robert Wood Johnson Foundation Grant Explorer, Grants to the American Cancer Society, 1972-2018, accessed June 20, 2018, https://rwjf.ws/2GpqunP.
- Robert Wood Johnson Foundation Grant Explorer Grant 59341, accessed June 12, 2018,
https://www.rwjf.org/en/how-we-work/grants-explorer.html#k=59341& sortBy=title&ascending=true.
- Robert Wood Johnson Foundation Grant Explorer Grant 59325 in 2006 ($92,300 to ACS’s Massachusetts Division) 2006; Grant 34190 ($332,000 to the Oregon Department of Human Services Public Health Division) 2004; Grant 41523 ($1.4 million to the Medical Society of New Jersey) 2001, https://rwjf.ws/2NrqAjH.
- Dani Veracity, “Is the American Cancer Society more interested in cancer profit than cancer prevention?” Natural News, July 31, 2005, https://www.naturalnews.com/010244_American_Cancer_Society_the_ACS.html.
- Cancer Action Network,” Tobacco Cessation Insurance Coverage,” August 7, 2017, https://www.acscan.org/policy-resources/tobacco-cessation-insurance-coverage.
- Campaign for Tobacco-Free Kids, “Kids Across America ‘Kick Butts’ On March 31,” news release, March 31, 2004, https://www.tobaccofreekids.org/press-releases/id_0738.
- Karen K. Gerlach and Michelle A. Larkin, “The Smokeless States Program,” Chapter Two of The Robert Wood Johnson Foundation Anthology: To Improve Health and Health Care, Volume VIII, p 8.
- Robert Wood Johnson Foundation Grant Explorer Grant 66857, accessed June 21, 2018,
https://www.rwjf.org/en/how-we-work/grants-explorer.html#k=66857& ascending=true.
- Robert Wood Johnson Foundation, “Robert Wood Johnson Foundation and Partners Launch New Public Health Law Initiative,” news release, September 21, 2010, https://www.rwjf.org/en/library/articles-and-news/2010/09/robert-wood- johnson-foundation-and-partners-launch-new-public-he.html.
Robert Wood Johnson Foundation Grant Explorer Grant 69789, accessed October 16, 2018, https://www.rwjf.org/en/how-we-work/grants-explorer.html.
- Truth Initiative Foundation, 2016 Return of Organization Exempt Form Income Tax (IRS Form 990), http://990s.foundationcenter.org/990_pdf_archive/911/911956621/911956621_ 201706_990.pdf.
- American Cancer Society, 2016 Return of Organization Exempt From Income Tax (IRS Form 990),
https://www.cancer.org/content/dam/cancer-org/online-documents/en/pdf/ policies/irs-form-990-2016.pdf.
- American Cancer Society Cancer Action Network, Inc., 2016 Return of Organization Exempt From income Tax (IRS Form 990), http://990s.foundationcenter.org/990_pdf_archive/522/522340031/522340031
_201612_990O.pdf.
- Campaign for Tobacco-Free Kids, 2016 Return of Organization Exempt From income Tax (IRS Form 990), http://990s.foundationcenter.org/990_pdf_archive/521/521969967/521969967
_201703_990.pdf.
- Tobacco-Free Kids Action Fund, 2016 Return of Organization Exempt From income Tax (IRS Form 990), http://990s.foundationcenter.org/990_pdf_archive/521/521974904/521974904
_201703_990O.pdf.
- American Lung Association, 2015 Return of Organization Exempt From income Tax (IRS Form 990), http://990s.foundationcenter.org/990_pdf_archive/131/131632524/131632524
_201606_990.pdf.
- Michael Johnsen, “Report: Nicotine replacement product sales to reach
$6.2 billion worldwide by 2018,” Drug Store News, August 16, 2012, https://www.drugstorenews.com/news/report-nicotine-replacement-product- sales-reach-62-billion-worldwide-2018.
- Associated Press, “Smoking rate in U.S. hits all-time low, CDC says,”
CBS News, June 19, 2018,
https://www.cbsnews.com/news/smoking-rate-in-u-s-hits-all-time-low.
- Centers for Disease Control and Prevention, “About Healthy People,” accessed June 21, 2018, https://www.healthypeople.gov/node/5840.
- Centers for Disease Control and Prevention, “Smoking is the leading cause of preventable death,” December 14, 2017, https://www.cdc.gov/winnablebattles/report/tobacco.html.
- Michael Grossman, Dhaval Dave, Henry Saffer, Don Kenkel, and Daniel Dench, “Should electronic cigarette producers be prohibited from advertising and be taxed?” VOX EU, Centre for Economic Policy Research, May 5, 2018, https://voxeu.org/article/should-e-cigarette-producers-be-prohibited- advertising-and-be-taxed.
- “Vape ’em if you got ’em,” The Economist, May 23, 2013, https://www.economist.com/news/business/21573985-challenge-big-tobacco- vape-em-if-you-got-em.
- Nancy Shute, “Health Organizations Call for a ban on E-Cigarettes Indoors,” National Public Radio, August 26, 2014,
https://www.npr.org/sections/health-shots/2014/08/26/343382661/health- organizations-call-for-a-ban-on-e-cigarettes-indoors.
Laura A. Bischoff, “Ban on sale of electronic cigarettes to minors passes Ohio Senate,” Dayton Daily News, February 12, 2014, https://www.daytondailynews.com/news/local/ban-sale-electronic-cigarettes- minors-passes-ohio-senate/ugWQ9GR8uxBJWTXemS8dZO.
- Family Smoking Prevention and Tobacco Control Act of 2009 Public Law 111-31, 111th Congress,
https://www.gpo.gov/fdsys/pkg/PLAW-111publ31/html/PLAW-111publ31.htm.
- Citizen Petition, “Asking the U.S. Food and Drug Administration to Assert Jurisdiction over and Regulate All Tobacco Products,” Tobacco Legal Consortium, September 6, 2013, http://bit.ly/2KmJn0C.
- Ban A.Majeed, Scott R.Weaver, Kyle R. Gregory, et al., “Changing Perceptions of Harm of E-Cigarettes among U.S. Adults, 2012–2015,” American Journal of Preventive Medicine, Vol. 52, Issue 3 (March 2017), pp. 331-338, https://www.sciencedirect.com/science/article/pii/S0749379716304433.
- Public Health England, “E-cigarettes: an evidence update,” August 19, 2015, https://www.gov.uk/government/publications/e-cigarettes-an-evidence-update.
- National Academies of Sciences, Engineering, and Medicine, “Public Health Consequences of E-Cigarettes,” Consensus Study Report, January 2018, https://www.nap.edu/catalog/24952/public-health-consequences-of-e-cigarettes.
- For more on IARC, see Angela Logomasini,” U.S. Should Stop Funding the International Agency for Research on Cancer,” OnPoint No. 248, Competitive Enterprise Institue, September 19, 2018,
https://cei.org/content/us-should-stop-funding-international-agency-research- cancer.
- Nigel Gray, Jack E Henningfield, Neal L Benowitz, et al., “Toward a comprehensive long term nicotine policy,” Tobacco Control, Vol. 14, No. 3 (2005), http://tobaccocontrol.bmj.com/content/14/3/161. Benowitz is a UCSF colleague of Stanton Glantz.
- Eric Lindblom, “Filling in the Blanks on Reducing Tobacco Product Addictiveness in the FCTC Partial Guidelines for Articles 9 & 10,” Working Paper No. 1, O’Neill Institute for National and Global Health Law, Georgetown Law Center, 2014, https://scholarship.law.georgetown.edu/cgi/viewcontent.cgi?article=2410& context=facpub.
- The snus available in the U.S. is not the same as snus available in Sweden.
- Joe Nocera, “Sweden Figured out How to Stop People from Smoking,” Bloomberg, June 8, 2017,
https://www.bloomberg.com/view/articles/2017-06-08/sweden-figured-out- how-to-stop-people-from-smoking.
- European Commission, “Attitudes of Europeans towards tobacco and electronic cigarettes,” Special Eurobarometer 458, May 2017, https://ec.europa.eu/commfrontoffice/publicopinion/index.cfm/ResultDoc/ download/DocumentKy/79002.
- Centers for Disease Control and Prevention, “Current Cigarette Smoking Among Adults in the United States,” Current Smoking Among Adults, 2016, https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/ index.htm.
- Nocera.
- The graph is from Lindsey A. Torre and Ahmedin Jemal, “Global trends of lung cancer mortality and smoking prevalence,” Translational Lung Cancer Research, Vol. 4, No. 4 (August 2015), pp. 327–338, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4549470.
It presents “incidence” and “mortality” as the same because “there are no substantial differences between incidence and mortality rates and trends, even in economically developed countries, because of poor survival.”
- Jaques Ferlay, Eve Steliarova-Foucher, Joannie Lortet-Tieulent, “Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012,” European Journal of Cancer, Vol. 49, Issue 6 (April 2013), https://www.sciencedirect.com/science/article/pii/S0959804913000075#!,.
- For a more in-depth discussion of the science used in regulating smokeless oral tobacco, see Christopher Snowdon, The Art of Suppression: Pleasure, Panic and Prohibition Since 1800 (North Yorkshire, UK: Little Dice, 2011), p. 150.
- Action on Smoking and Health is now a global organization, with members in many countries. “About ASH,” ASH website, accessed October 16, 2018, https://ash.org/about/.
- Snowdon, The Art of Suppression, pp. 145-149. By 2004, ASH admitted the science originally used to justify the snus ban was not relevant to snus and argued for the product’s legalization and regulation.
- UK Parliament, The Oral Snuff (Safety) Regulations, 1989, No. 2347, http://www.legislation.gov.uk/cy/uksi/1989/2347/made/data.xht?view=snippet &wrap=true.
- Snowdon, The Art of Suppression, pp. 145-149.
- European Commission, Tobacco Products Directive, Directive 2001/37/EC of the European Parliament and of the Council, June 5, 2001, https://ec.europa.eu/health/sites/health/files/tobacco/docs/dir200137ec_ tobaccoproducts_en.pdf.
- Carl V. Phillips, “Gateway Effects: Why the Cited Evidence Does Not Support Their Existence for Low-Risk Tobacco Products (and What Evidence Would),” International Journal of Environmental Research and Public Health, Vol. 12, No. 5 (May 2015), pp. 5439–5464, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4454978.
- Email from Clive Bates, “Re: [GLOBALink] New Scientist on harm reduction,” November 8, 2001, accessed June 20, 2018, https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=htdn0191.
- Snowdon, The Art of Suppression, p. 155.
- University of California, San Francisco, Cardiology, “Faculty Spotlight: Dr. Stanton Glantz,” accessed June 10, 2018, https://cardiology.ucsf.edu/facstaff/spotlight/glantz.html.
- Gene Healy, “The Era of Jackbooted Nags,” Commentary, Cato Institute, June 3, 2005, https://www.cato.org/publications/commentary/era-jackbooted-nags.
- Anne Landman and Stanton Glantz, “Tobacco Industry Efforts to Undermine Policy-Relevant Research,” American Journal of Public Health, Vol. 99, No. 1 (January 2009), https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2600597.
- Stanton A Glantz and L. R. Smith, “The effect of ordinances requiring smoke- free restaurants on restaurant sales,” American Journal of Public Health, Vol. 84, No. 7 (1994), pp. 1081–1085, https://ajph.aphapublications.org/doi/pdfplus/10.2105/AJPH.84.7.1081.
- Centers for Disease Control and Prevention, “Smokefree Policies Do Not Hurt the Hospitality Industry,” December 1, 2016, accessed October 17, 2018, https://www.cdc.gov/tobacco/data_statistics/fact_sheets/secondhand_smoke/ protection/hospitality/index.htm.
American Cancer Society, “The Facts About Secondhand Smoke” October 2014, http://bit.ly/ACSsecond.
- Michael K. Evans, “A review of the effect of ordinances requiring smoke-free restaurants on restaurant sales’ by Stanton A. Glantz and Lisa R.A. Smith,” Report by the Evans Group for the National Smokers’ Alliance, March 1997.
- Bennett and DiLorenzo, From Pathology to Politics, p. 109.
- Robert Wood Johnson Foundation, Grants Explorer, accessed October 29, 2018, https://www.rwjf.org/en/how-we-work/grants-explorer.html#k= restaurants%20indoor&start=1997&end=2002.
- Betty Durston and Konrad Jamrozik, eds., “Tobacco & health 1990: the global war: proceedings of the Seventh World Conference on Tobacco and Health,” Perth, Western Australia, April 1-5, 1990, https://trove.nla.gov.au/work/6558889?q&versionId=7559786.
- Christopher Snowdon, Velvet Glove, Iron Fist: A History of Anti-Smoking, (London; United Kingdom; 2009), loc 3530.
- Victoria MacDonald, “Passive smoking doesn’t cause cancer—official,”
Sunday Telegraph, March 8, 1998.
- Richard Hannaford, “UK Paper under fire for smoking story,” BBC News, March 27, 1998, http://news.bbc.co.uk/2/hi/uk_news/70433.stm. “Smokescreens—The World Health Organization is showing signs of allowing politics to get in the way of truth,” The Economist, March 14, 1998, http://bit.ly/2K6pETv).
- Christopher Snowdon, “Velvet Glove, Iron Fist,” loc. 5081, 5148.
- Richard P. Sargent, Robert M. Shepard, and Stanton A. Glantz, “Reduced incidence of admissions for myocardial infarction associated with public smoking ban: before and after study,” British Medical Journal, Vol. 238, No. 7446 (April 2004), pp. 977-980, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC404491/?tool=pmcentrez& report=abstract.
- Richard P. Sargent, “Immediate Reduction in Acute Myocardial Infarctions after Implementation of a Comprehensive Smokefree Ordinance,” PowerPoint presen- tation to the American College of Cardiology 52nd Annual Scientific Sessions, Chicago, April 2, 2003, https://www.slideserve.com/andrew/heart-attacks.
- Sargent, Shepard, and Glantz, “Reduced incidence of admissions for myocar- dial infarction associated with public smoking ban: before and after study.”
- David Harsanyi, Nanny State: How Food Fascists, Teetotaling Do-Gooders, Priggish Moralists, and other Boneheaded Bureaucrats are Turning America into a Nation of Children (New York : Broadway Books, 2007).
- Brad Rodu and Philip Cole, “Helena Study Random Variation Confirmed,” BMJ Rapid Responses, January 14, 2006, https://www.bmj.com/content/328/7446/977/rapid-responses.
- University of Louisville “Bucks for Brains: Endowed Chair in Tobacco Harm Reduction Research,” accessed October 16, 2018, http://louisville.edu/bucksforbrains/descriptions/tobaccoharmreduction.
- Brad Rodu and Philip Cole, “Additional Information on Acute Myocardial Infarctions in Helena, Montana” British Medical Journal, Rapid Responses, Vol. 328, (2004),
https://www.bmj.com/rapid-response/2011/10/30/additional-information- acute-myocardial-infarctions-helena-montana.
- Christopher Snowdon, “Heart miracles: is the truth emerging?” Velvet Glove Iron Fist, October 20, 2014, https://velvetgloveironfist.blogspot.com/2014/10/heart-miracles-is-truth- emerging.html.
- Henry F. Mizgala, “Science Blinded by Wishfull Thinking,” BMJ Rapid Responses, Vol. 328, (2004),
https://www.bmj.com/rapid-response/2011/10/30/science-blinded-wishfull- thinking.
Mizgala does not appear to have financial ties to the tobacco, smokeless tobacco, or vaping industries, though Philip Morris relied on his opinion as evidence in a 1998 lawsuit, Iron Workers Local Union No. 17 Ins. Fund v. Philip Morris Inc. 29 F. Supp. 2d 801 (N.D. Ohio 1998), https://law.justia.com/cases/federal/district-courts/FSupp2/29/801/2472144/.
- Geoffrey C. Kabat, “When results look too good to be true, they probably are,”
BMJ Rapid Responses, Vol. 328, (2004),
https://www.bmj.com/rapid-response/2011/10/30/when-results-look-too-good- be-true-they-probably-are.
- Rosemary Ellis, “The Secondhand Smoking Gun,” New York Times, October 15, 2003,
https://www.nytimes.com/2003/10/15/opinion/the-secondhand-smoking-gun.html.
- Jeremy Laurance, “Smoking ban could halve number of heart attacks: first edition,” The Independent, April 5, 2004.
- Marc Kaufman, “Secondhand Smoke Poses Heart Attack Risk, CDC Warns,” Washington Post, April 23, 2004, https://www.washingtonpost.com/archive/politics/2004/04/23/secondhand- smoke-poses-heart-attack-risk-cdc-warns/5cd7575f-e9e7-4454-b816- 11c6881d2a86/?noredirect=on&utm_term=.3e06304bffaa.
Centers for Disease Congrol and Prevention, 2006 Surgeon General’s Report— The Health Consequences of Involuntary Exposure to Tobacco Smoke, https://www.cdc.gov/tobacco/data_statistics/sgr/2006/index.htm.
- Edie Lau, “UCLA smoking study criticized,” Sacramento Bee, May 16, 2003.
- Sheldon Ungar and Dennis Bray, “Silencing science: partisanship and the career of a publication disputing the dangers of secondhand smoke,” Public Understanding of Science, Vol. 14, Issue 1 (2005), pp. 5-23, http://journals.sagepub.com/doi/abs/10.1177/0963662505048515.
- Sarah Baldauf, “Caution: Don’t Assume ‘Smokeless’ Substitutes for Cigarettes Are Safe,” U.S. News & World Report, January 13, 2009, https://health.usnews.com/health-news/family-health/articles/2009/01/13/ caution-dont-assume-smokeless-substitutes-for-cigarettes-are-safe.
- Duff Wilson and Julie Creswell,” Where There’s Smoke, Altria Hopes There’s Fire,” New York Times, January 30, 2010, https://www.nytimes.com/2010/01/31/business/31altria.html.
Stanton Glantz Tweet, October 1, 2013 7:34 am, https://twitter.com/ProfGlantz/status/384891459230392320.
- Sabrina Tavernise, “A Hot Debate over E-Cigarettes as a Path to Tobacco, or from It,” New York Times, February 22, 2014,
https://www.nytimes.com/2014/02/23/health/a-hot-debate-over-e-cigarettes-as- a-path-to-tobacco-or-from-it.html.
- Stanton A. Glantz, “First evidence of long-term health damage from ecigs: Smoking E-Cigarettes Daily Doubles Risk of Heart Attacks,” University of California, San Francisco, Center for Tobacco Control Research and Education, news release, February 24, 2018,
https://tobacco.ucsf.edu/first-evidence-long-term-health-damage-ecigs-smoking- e-cigarettes-daily-doubles-risk-heart-attacks.
- Here & Now, “Vaping’s Popularity—Especially Among Teens— Is Cause For Concern, Researcher Says,” WBUR, May 16, 2018,
http://www.wbur.org/hereandnow/2018/05/16/vaping-e-cigarettes-use-teens.
- Wang et al.
- Phillips.
- Rachel Russell, “E-cigarettes revealed to be ‘gateway out of tobacco smoking’ for young people,” Harrow Times, September 6, 2017.
- Jia Tolentino, “The Promise of Vaping and the Rise of Juul” The New Yorker, May 14, 2018,
https://www.newyorker.com/magazine/2018/05/14/the-promise-of-vaping- and-the-rise-of-juul.
- Jonathan P. Winickoff, Elyse R. Park, Bethany J. Hipple, Anna Berkowitz, Cecilia Vieira, Joan Friebely, Erica A. Healey, and Nancy A Rigotti, “Clinical Effort Against Secondhand Smoke Exposure (CEASE): Development of Framework and Intervention,” Pediatrics, Vol. 122, No. 2 (August 2008),
pp. e363-e375, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2774730/.
- Michael Siegel, “E-Cigarette Opponents Still Making Up False ‘Facts’ to Demonize Vaping,” The Rest of the Story: Tobacco Analysis and Commentary, May 8, 2018, http://tobaccoanalysis.blogspot.com/2018/05/e-cigarette-opponents-still- making-up.html.
- Winickoff devised the concept of “thirdhand smoke,” the idea that gases and particles from smoking cling to users’ hair and clothes, seep into carpets, and harm babies and toddlers. His alleged evidence behind the theory came from a telephone survey that asked people if they believed thirdhand smoke was harmful to their children. He has advocated for bans on smoking in public housing and evicting residents who do not comply. Regulation of Jonathan P. Winickoff, Mark Gottlieb, and Michelle M. Mello, “Smoking in Public Housing,” New England Journal of Medicine, Vol. 362, No. 24 (June 17, 2010), https://www.nejm.org/doi/full/10.1056/NEJMhle1000941.
- Sara Kalkhoran and Stanton Glantz, “E-cigarettes and smoking cessation in real-world and clinical settings: a systematic review and meta-analysis,” The Lancet Respiratory Medicine, Vol. 4, No. 2 (February 2016), https://www.ncbi.nlm.nih.gov/pubmed/26776875.
- “Expert reaction to meta-analysis looking at e-cigarette use and smoking cessation,” Science Media Centre, January 14, 2016, http://www.sciencemediacentre.org/expert-reaction-to-meta-analysis-looking- at-e-cigarette-use-and-smoking-cessation.
- Ibid.
- M. David Dobbins, Letter to FDA Acting Commissioner Stephen Ostroff, July 2, 2015,
http://healthdocbox.com/amp/78633164-Smoking_Cessation/July-2-re-docket- no-fda-2014-n-dear-dr-ostroff.html.
- Lisa Rapaport, “E-cigarettes tied to less smoking cessation,” Reuters, March 22, 2018,
https://www.reuters.com/article/us-health-smoking-quitting-ecigarettes/ e-cigarettes-tied-to-less-smoking-cessation-idUSKBN1GY2P8.
- Here & Now, “Vaping’s Popularity—Especially Among Teens—Is Cause For Concern, Researcher Says,” WBUR, May 16, 2018, http://www.wbur.org/hereandnow/2018/05/16/vaping-e-cigarettes-use-teens.
- Nathan Gale, Mike McEwan, Alison C. Eldridge, et al., “Changes in Biomarkers of Exposure on Switching From a Conventional Cigarette to Tobacco Heating Products: A Randomized, Controlled Study in Healthy Japanese Subjects,” Nicotine & Tobacco Research, June 15, 2018, https://www.ncbi.nlm.nih.gov/pubmed/29912406.
- Michael Siegel, “More Conflicts of Interest Being Hid by Electronic Cigarette Opponents: Funding of their Organization by Big Pharma Not Disclosed,” Tobacco Analysis, August 31, 2012, http://tobaccoanalysis.blogspot.com/2012/08.
- David Kesmodel, “Glaxo Aims to Snub out ‘Dissolvable’ Tobacco Items,” Wall Street Journal, September 28, 2010, https://www.wsj.com/articles/SB10001424052748703694204575518294130892622. Makiki Kitamura, “Glaxo Memo Shows Drug Industry Lobbying on E-Cigarettes,” Bloomberg, February 19, 2014, https://www.bloomberg.com/news/articles/2014-02-19/glaxo-memo-shows- drug-industry-lobbying-on-e-cigarettes.
- Campaign for Tobacco-Free Kids, “Mints, Gum, Candy—or Tobacco?” Tobacco Unfiltered Blog, January 20, 2012, https://www.tobaccofreekids.org/blog/2012_01_20_yaya.
- Bruce Yandle, “Bootleggers and Baptists—The Education of a Regulatory Economist,” Regulation, May/June 1983, pp. 12-16, https://techliberation.com/wp-content/uploads/2010/12/v7n3-3.pdf.
- Bruce Yandle, “Bootleggers and Baptists in Retrospect, Regulation, Vol. 22, No. 3 (Fall 1999), https://object.cato.org/sites/cato.org/files/serials/files/regulation/1999/10/ bootleggers.pdf.
- Jonathan H. Adler, Roger E. Meiners, Adnrew P. Morriss, and Bruce Yandle, “Baptists, Bootleggers & Electronic Cigarettes,” Yale Journal on Regulation, Vol. 33, No. 2, (2016).
- Ibid.
- American Legacy Foundation and Affiliate, “Consolidated Financial Report,” June 30, 2015, accessed June 1, 2018, http://bit.ly/2ttzxk2. The Truth Initiative also funds anti-tobacco activism. For example, in 2003 the Robert Wood Johnson Foundation created the Smoking Cessation Leadership Center (SCLC) at UCSF with a five-year grant. SCLC later received “significant funding” from the Truth Initiative. UCSF Smoking Cesation Leadership Center “About SCLC,” accessed June 20, 2018, https://smokingcessationleadership.ucsf.edu/about. In August 2007, the Truth Initiative (then the American Legacy Foundation) gave UCSF a donation to name Stanton Glantz as the Distinguished Professor of Tobacco Control. UCSF Today, “American Legacy Foundation Honors Glantz with Distinguished Professorship,” news release, August 15, 2007, http://bit.ly/2yGXWaV. The Master Settlement Agreement called for the American Legacy Foundation to be formed with an initial board of 11 individuals, with two members appointed each by the National Association of Attorneys General, the National Governors Association, and the National Conference of State Legislatures. The remaining five board members were appointed by the first six appointees.
- States spend less than 2 cents from each dollar they receive from the settlement agreement on tobacco harm reduction. Thomas Carr, “Who Is Really Benefiting From the Tobacco Settlement Money?” American Lung Association,
February 3, 2016 (updated March 16, 2018),
http://www.lung.org/about-us/blog/2016/02/who-benefit-tobacco-settlement.html.
- Samuel Loewenberg, “Smoke Screen: Why is Philip Morris Supporting FDA Regulation of Cigarettes?” Slate, July 25, 2002, http://www.slate.com/articles/business/moneybox/2002/07/smoke_screen.html.
- Duff Wilson, “Philip Morris’s Support Casts Shadow over a Bill to Limit Tobacco,” New York Times, March 31, 2009, https://www.nytimes.com/2009/04/01/business/01tobacco.html.
- For example, warnings changed from a “Surgeon General’s Warning” that “Smoking causes lung cancer, heart disease, emphysema, and may complicate pregnancy,” and “Quitting smoking now greatly reduces serious risks to your health,” to “Warning: Cigarettes are addictive,” “Tobacco smoking can harm your children,” “Cigarettes cause fatal lung disease,” “Cigarettes cause cancer,” and “Cigarettes cause strokes and heart disease.”
- Monica Showalter, “Hot Air: Democrats Work With Big Tobacco and Big Pharma to Choke the Vaping Industry,” The Observer, June 2, 2016, http://observer.com/2016/06/hot-air-democrats-work-with-big-tobacco-and- big-pharma-to-choke-the-vaping-industry.
Jonathan H. Adler, “‘Baptists, Bootleggers & Electronic Cigarettes’: A response to Professor Berman,” Washington Post, July 10, 2016,
https://www.washingtonpost.com/news/volokh-conspiracy/wp/2016/07/10/ baptists-bootleggers-electronic-cigarettes-a-response-to-professor-berman/
?utm_term=.ce5cb0d2dfad.
- Mike Enzi, “HELP Committee Passes a ‘Marlboro Protection Act,’”
The Hill, August 1, 2007,
http://thehill.com/blogs/congress-blog/politics/27745-help-committee-passes- a-marlboro-protection-act-sen-mike-enzi.
- Altria Client Services to Margaret A. Hamburg, October 10, 2013, http://www.altria.com/About-Altria/Federal-Regulation-of-Tobacco/Regulatory
-Filing/FDAFilings/Support-of-Unregulated-Tobacco-%20Products.pdf. Deeming Tobacco Products to Be Subject to the Federal Food, Drug, and Cosmetic Act, as Amended by the Family Smoking Prevention and Tobacco Control Act; Regulations on the Sale and Distribution of Tobacco Products and Required Warning Statements for Tobacco Products; Extension of Comment Period, Federal Register, Vol. 79, No. 121 (Tuesday, June 24, 2014), re Docket
No. FDA-2014-N-0189, June 24, 2014,
https://www.federalregister.gov/documents/2014/06/24/2014-14562/deeming- tobacco-products-to-be-subject-to-the-federal-food-drug-and-cosmetic-act-as- amended-by-the.
- U.S. Department of Health and Human Services, “Deeming Tobacco Products to Be Subject to the Federal Food, Drug, and Cosmetic Act, as Amended by the Family Smoking Prevention and Tobacco Control Act; Regulations on the Sale and Distribution of Tobacco Products and Required Warning Statements for Tobacco Products,” Preliminary Regulatory Impact Analysis, Initial Regulatory Flexibility Analysis, Unfunded Mandates Reform Act Analysis, Docket No. FDA-2014-N-0189, April 2014, https://www.fda.gov/downloads/AboutFDA/ReportsManualsForms/Reports/ EconomicAnalyses/UCM394933.pdf.
- Meg Neal, “The Maker of Vuse E-Cigs Is Lobbying to Ban Vaping,” Gizmodo, September 11, 2014,
https://gizmodo.com/the-maker-of-blu-e-cigs-is-lobbying-to-ban-vaping- 1633442788.
- Campaign for Tobacco-Free Kids, “The Flavor Trap: how tobacco companies are luring kids with candy-flavored e-cigarettes and cigars,” March 15, 2017, https://www.tobaccofreekids.org/microsites/flavortrap.
- René A. Arrazola, Shanta R. Dube, and Brian A. King, “Tobacco Product Use Among Middle and High School Students—United States, 2011 and 2012,” Morbidity and Mortality Weekly Report, Centers for Disease Control and Prevention, November 15, 2013, https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6245a2.htm.
- Joe Nocera, “Smoking, Vaping and Nicotine,” New York Times, May 26, 2015, https://www.nytimes.com/2015/05/26/opinion/joe-nocera-smoking-vaping- and-nicotine.html.
- Jack E Henningfield, Christine A Rose, and Mitch Zeller, “Tobacco industry litigation position on addiction: continued dependence on past views,” Tobacco Control, Vol. 15, Suppl. 4 (December 15, 2006), pp. iv27-iv36, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2563585.
Richard Craver, “FDA names new head of tobacco governance group,” Winston-Salem Journal, February 23, 2013, https://www.journalnow.com/business/business_news/national_international/ fda-names-new-head-of-tobacco-governance-group/article_b47cf1b6-7d56- 11e2-81dc-001a4bcf6878.html.
- Wendy Koch, “E-cigarette use doubles among U.S. teens,” USA Today, September 5, 2013, https://www.usatoday.com/story/news/nation/2013/09/05/e-cigarette-use- doubles-among-us-teens/2768155/.
- While a single year is not enough to establish a trend (either increasing or decreasing), the fact that CDC found a decrease among adolescent use of combustible tobacco in this and subsequent years defies Myers’s assertion. Centers for Disease Control and Prevention, “Tobacco Product Use Among Middle and High School Students—United States, 2011 and 2012.”
- Rachel Grana, Neal Benowitz, and Stanton A. Glantz, “E-Cigarettes: A Scientific Review,” Circulation, Vol. 129, No. 19 (May 13, 2014), pp. 1972-1986, https://www.ncbi.nlm.nih.gov/pubmed/24821826.
- Karen Kaplan and Eryn Brown, “Teen use of e-cigarettes continues to soar, alarming many experts,” Los Angeles Times, April 16, 2015, http://www.latimes.com/science/la-sci-sn-smoking-tobacco-use-students- 20150415-story.html.
- Adam Leventhal, David Strong, Matthew Kirkpatrick, et al., “Association of electronic cigarette use with initiation of combustible tobacco product smoking in early adolescence,” Journal of the American Medical Association, Vol. 413, No. 7 (2015), pp. 700-707, https://jamanetwork.com/journals/jama/fullarticle/2428954.
- Maggie Fox, “Teenagers Who Try E-Cigarettes Also Try Smoking, Study Finds,” NBC News, August 18, 2015, https://www.nbcnews.com/health/kids-health/teens-who-try-e-cigs-try- smoking-too-study-finds-n411871.
- Mike Stobbe, “Study finds most teens vaping fruity flavors, not nicotine,” Associated Press, August 25, 2016, https://apnews.com/6e8a850ba66946349f99952e55a76494.
- Richard Miech, Megan E. Patrick, Patrick M. O’Malley, and Lloyd D. Johnston, “What are kids vaping? Results from a national survey of US adolescents,” Tobacco Control, Vol. 26, No. 4 (2017), pp. 386-391, http://tobaccocontrol.bmj.com/content/26/4/386.info.
- Tripp Mickle, “Study Finds Ninth-Graders Who Used E-Cigarettes More Likely to Smoke,” Wall Street Journal, August 18, 2015, https://www.wsj.com/articles/study-finds-ninth-graders-who-used-e-cigarettes- more-likely-to-smoke-1439910081.
- Bradley J. Fikes, “Vaping-friendly legislation killed by Congress,” San Diego Union Tribune, May 1, 2017, http://www.sandiegouniontribune.com/business/biotech/sd-me-cole-bishop- 20170501-story.html.
- Food and Drug Administration, Notice of Proposed Rulemaking, “Deeming Tobacco Products to Be Subject to the Federal Food, Drug, and Cosmetic Act, as Amended by the Family Smoking Prevention and Tobacco Control Act; Regulations on the Sale and Distribution of Tobacco Products and Required Warning Statements for Tobacco Products,” Docket No. FDA-2014-N-0189, April 25, 2014,
https://www.federalregister.gov/documents/2014/04/25/2014-09491/deeming- tobacco-products-to-be-subject-to-the-federal-food-drug-and-cosmetic-act-as- amended-by-the.
The “deeming rule” was delayed in July 2017 by newly appointed FDA Com- missioner Scott Gottlieb. It is now set to go into effect in 2022.
- U.S. Food and Drug Administration, Tobacco Product Marketing Orders, https://www.fda.gov/TobaccoProducts/Labeling/TobaccoProductReviewEvaluation
/ucm339928.htm.
- David Nather and Dylan Scott, “FDA issues sweeping regulations for e-cigarettes for first time,” STAT News, May 5, 2016, https://www.statnews.com/2016/05/05/e-cigarette-cigar-tobacco-rule-released.
- Ibid.
- Allison Glasser, Haneen Abudayyeh, Jennifer Cantrell, and Raymond Niaura, “Patterns of E-Cigarette Use Among Youth and Young Adults: Review of the Impact of E-Cigarettes on Cigarette Smoking,” Nicotine & Tobaco Research, May 17, 2018, https://academic.oup.com/ntr/advance-article-abstract/doi/ 10.1093/ntr/nty103/4998820.
- Ahmed Jamal, Andrea Gentzke, S. Sean Hu, Karen A. Cullen, Benjamin J. Apelberg, David M. Homa, and Brian A. King, “Tobacco Use Among Middle and High School Students—United States, 2011–2016,” Morbidity and Mortality Weekly Report, Centers for Disease Control and Prevention, June 16, 2017, https://www.cdc.gov/mmwr/volumes/66/wr/mm6623a1.htm#F1_down.
- Campaign for Tobacco-Free Kids, “The Flavor Trap.”
- Campaign for Tobacco-Free Kids, “E-Cigarette Marketing Continues to Mirror Cigarette Marketing,” Tobacco Unfiltered Blog, June 17, 2015, https://www.tobaccofreekids.org/blog/2015_06_17_ecig.
- Richard Craver, “Juul surpasses Vuse in electronic-cigarette sales,” Winston-Salem Journal, November 17, 2017, http://www.journalnow.com/business/juul-surpasses-vuse-in-electronic- cigarette-sales/article_e6266762-0290-54b3-926d-74ed4e989507.html.
- Campaign for Tobacco-Free Kids, “JUUL and yoth: rising e-cigarette popularity,” news release, January 9, 2018, https://bit.ly/2HgH0er. This release has been reissued at least three times, on February 5, 2018; April 4, 2018; and
April 17, 2018.
- Truth Initiative, “What is Juul?” news release, February 5, 2018, https://truthinitiative.org/news/what-is-juul.
- Caroline Kee, “Everything You Need to Know about the JUUL, including the Health Effects,” BuzzFeed News, February 5, 2018, https://www.buzzfeed.com/carolinekee/juul-ecigarette-vape-health- effects?utm_term=.utrO7dw5k#.gtooZM7Nb.
- Google search, terms “juul,” “crème brulee” -brûlée -creme -brulée
-tobaccofreekids –truthinitiative,” http://bit.ly/2K5LeaM.
- Ana B. Ibarra, “Juul e-cigarettes and teens: Health problem of the decade’?” CNN, March 15, 2018,
https://www.cnn.com/2018/03/15/health/juul-e-cigarette-partner/index.html.
- Email conversation with Ann B. Ibarra April, 13, 2018.
- Campaign for Tobacco Free Kids, “Leading Health Organizations Seek to Intervene in Defense of FDA Rule on E-Cigarettes, Cigars,” news release, July 24, 2017,
https://www.tobaccofreekids.org/press-releases/2017_07_24_fda.
- Roni Selig, “Vaping is now an epidemic among U.S. high school students,” CNN, April 6, 2018,
https://www.cnn.com/2018/04/06/health/high-schools-vaping-epidemic/index.html.
- Ahmed Jamal, Andrea Gentzke, S. Sean Hu, Karen A. Cullen, Benjamin J. Apelberg, David M. Homa, and Brian A. King, “Tobacco Use Among Middle and High School Students—United States, 2011–2016,” Morbidity and Mortality Weekly Report, Centers for Disease Control and Prevention, June 16, 2017, https://www.cdc.gov/mmwr/volumes/66/wr/mm6623a1.htm#F1_down.
- Ibid.
- Laura Bach, “JUUL and Youth: Rising E-Cigarette Popularity,” Campaign for Tobacco-Free Kids Fact Sheet, June 12, 2018, https://www.tobaccofreekids.org/assets/factsheets/0394.pdf.
- Matt (reviewer pen name), “JUUL Coupon Code and Starter Kit Review” Vape Pen Starter Kit, August 18, 2017, http://www.vapepenstarterkit.com/juul-coupon-code/.
- Washington Department of Health, “Vapor Products,” March 23, 2018, accessed June 21, 2018, https://www.doh.wa.gov/YouandYourFamily/Tobacco/VaporProducts.
- Division of Public Health, Delaware Department of Health and Social Services, “DPH Advises Parents, Teachers of New E-cigarette Trend among Teens: Juuling,” news release, April 12, 2018, http://dhss.delaware.gov/dhss/pressreleases/2018/ecigarette_041218.html.
- Pallone to Gottlieb: “JUULing” Threatens Kids’ Health,” March 9, 2018, https://pallone.house.gov/media/press-releases/pallone-gottlieb-juuling- threatens-kids-health.
- Office of Sen. Charles E. Schumer, “Schumer Standing with Teens Who Admit They’re Addicted to E-cig Juul, demans Feds Use Law on Books to Outright Ban Kid-Friendly Flavors in all E-cig Devices,” news release, May 6, 2018, https://www.schumer.senate.gov/newsroom/press-releases/schumer-standing- with-teens-who-admit-theyre-addicted-to-e-cig-juul-demands-feds-use-law- on-books-to-outright-ban-kid-friendly-flavors-in-all-e-cig-devices-with-kid-like
-tastes-in-every-puff-popularity-of-devices-has-now-metastasized-to-middle- schools-slow-moving-rules-on-flavors-masked-as-whipped-cream-or-candy-is- recipe-for-disaster.
- Email from Laura Bach, Campaign for Tobacco-Free Kids to State Program Contacts, “JUUL in schools,” December 19, 2017, obtained via Freedom of Information Act request, April 18, 2018.
- Email from Susan Weisman to Jeffrey Willett, Erika Mansur, April Roeseler, obtained via Freedom of Information Act request, February 15, 2018.
- Campaign for Tobacco-Free Kids, “E-Cigarettes Should be Included in Smoke-Free Laws,” CTFK Fact Sheet, March 8, 2018, https://www.tobaccofreekids.org/assets/factsheets/0387.pdf.
- Campaign for Tobacco-Free Kids, “Health Groups File Suit to Expedite FDA Review of E-Cigarettes, Cigars,” news release, March 27, 2018, https://www.tobaccofreekids.org/press-releases/2018_03_27_fdalawsuit.
- Ohio Department of Education, “Know! Warning—JUUL at Your School,” news release, March 21, 2018, https://bit.ly/2k9PtD4. Washington State Department of Health, “Vapor products,” March 23, 2018, https://bit.ly/2Jkj0V0.
Virginia Department of Behavioral Health and Developmental Services, “ Webinar: What’s the Hype?: JUUL Electronic Cigarette’s Popularity with Youth & Young Adults,” March 26, 2018, https://bit.ly/2Iulm3x.
- Centers for Disease Control and Prevention, “Teachers and Parents: That USB Stick Might be an E-cigarette,” April 15, 2018, http://bit.ly/2KPZK6m.
- Campaign for Tobacco-Free Kids, “Leading Health and Medical Groups Urge Immediate FDA Action to Address Rising Youth Use of Juul E-Cigarettes,” news release, April 18, 2018,
https://www.tobaccofreekids.org/press-releases/2018_04_18_juul.
- Sen. Richard J. Durbin, Sen. Richard Blumenthal, Sen. Sherrod Brown, et al., Letter to Scott Gottlieb, April 18, 2018, https://www.durbin.senate.gov/imo/media/doc/FDA%20Letter%20-
%20SIGNED.pdf.
- Laurie McGinley, “FDA cracks down on sales of Juul, other e-cigarettes to youths,” Washington Post, April 24, 2018, https://www.washingtonpost.com/news/to-your-health/wp/2018/04/24/fda-cracks- down-on-sales-of-juul-other-e-cigarettes-to-youth/?noredirect=on&utm_term=. 51271028d709.
- Statement of Matthew L. Myers, President, Campaign for Tobacco-Free Kids, “FDA Takes Important Steps to Address Youth Use of Juul E-Cigarettes, but Must Do More—Agency Should Prevent Introduction of Kid-Friendly Tobacco Products Instead of Acting After the Fact,” April 24, 2018, https://newsroom.heart.org/news/fda-takes-important-steps-to-address-youth- use-of-juul-e-cigarettes-but-must-do-more-agency-should-prevent-introduction- of-kid-friendly-tobacco-products-instead-of-acting-after-the-fact.
- Shia Levitt, “San Francisco to Vote on Ban of Flavored E-Cigarettes and Tobacco,” National Public Radio, May 6, 2018, https://www.npr.org/2018/05/06/608868114/san-francisco-to-vote-on-ban-of- flavored-e-cigarettes-and-tobacco.
- Stanton Glantz, “R.J. Reynolds Tobacco continues to be sole funder of Prop E, the company’s effort to repeal San Francisco’s prohibition on selling flavored tobacco products,” UCSF Center for Tobacco Control Research and Education, April 28, 2018,
https://tobacco.ucsf.edu/rj-reynolds-tobacco-continues-be-sole-funder-prop-e- company%E2%80%99s-effort-repeal-san-francisco%E2%80%99s-prohibition- selling-flavored-tobacco-products.
- Bloomberg Associates Foundation, 2016 Return of Private Foundation (IRS form 990), http://990finder.foundationcenter.org/990results.aspx?990_type=&fn=bloomberg &st=&zp=&ei=&fy=&action=Search.
- California Department of Health, “New Campaign Targets Tobacco Industry’s Deceptive Marketing to Youth,” news release, April 24, 2018, https://www.cdph.ca.gov/Programs/OPA/Pages/NR18-022.aspx.
- California Tobacco Control Program Funding Opportunities and Resources, Solicitation RFP 17-10048 California Tobacco Control Advertising Campaign, https://tcfor.catcp.org/index.cfm?fuseaction=opportunities.viewArchivedOpp &oppID=53.
Jim McDonald, “California Will Pay Kids to Vape in Propaganda Videos,” Vaping360, January 10, 2018.
- San Francisco, California, Proposition E, Ban on the Sale of Flavored Tobacco (June 2018), Ballotpedia, accessed June 15, 2018, https://ballotpedia.org/San_Francisco,_California,_Proposition_E,_Ban_on_the_ Sale_of_Flavored_Tobacco_(June_2018).
- Campaign for Tobacco-Free Kids, Truth Initiative, American Academy of Pediatrics, American Cancer Society, Cancer Action Network, American Heart Association, and American Lung Association, Coalition letter to Scott Gottlieb, “Need for immediate FDA action to protect young people from Juul
electronic cigarettes,” April 18, 2018, https://bit.ly/2rVnvyl.
- Statement of Matthew L. Myers, “Study Finds Teens Can Easily Buy E-Cigarettes Online—FDA Must Act to Protect Kids,” Campaign for Tobacco-Free Kids, March 2, 2015,
https://www.tobaccofreekids.org/press-releases/2015_03_02_ecig.
- Prior to his confirmation as FDA commissioner, Scott Gottlieb held a board seat on the vapor company Kure. Lydia Wheeler, “FDA to launch campaign warning kids of e-cigarette dangers,” The Hill, August 8, 2017, http://thehill.com/regulation/pending-regs/345740-fda-to-launch-campaign- warning-kids-of-e-cigarette-dangers.
- Brad Rodu, “UK Favors E-Cigarettes, Scores Major Smoking Reduction; Anti- Vaping Ireland Sees Smoking Rates Unchanged,” Tobacco Truth, August 15, 2018, https://rodutobaccotruth.blogspot.com/2018/08/uk-favors-e-cigarettes-scores- major.html.
- U.S. Food and Drug Administration, “Statement from FDA Commissioner Scott Gottlieb, M.D., on new steps to address epidemic of youth e-cigarette use.”