For over three decades, CEI has advocated health care reforms that put more power in the hands of consumers to choose their health providers, treatment protocols, and scope of insurance coverage. We have advocated reform of the Food and Drug Administration’s drug and device approval process to allow for greater flexibility and patient choice. And in 2013, CEI organized the court challenges to Obamacare’s exchange subsidies that concluded with the Supreme Court’s King v. Burwell decision.
Healthcare Issue Areas
Featured Posts

News Release
Rescheduling marijuana to a less restrictive category will benefit liberty and public health
President Trump signed an executive order today intended to fast track the rescheduling of cannabis from a Schedule I substance, the same as heroin,…

News Release
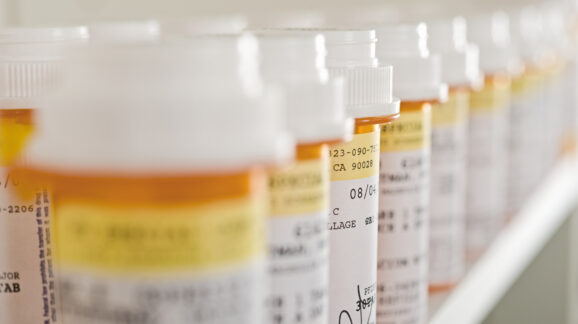
CEI Study: Government laws against pharmacy ownership would increase costs, restrict access for consumers
Innovation is reshaping how Americans get their prescription medications. Whether picking up medications in person, ordering online, or getting same-day delivery from services like Capsule,…
Study
A Free Market is the Best Medicine
Introduction The pharmaceutical supply market is seeing extraordinarily high levels of innovation and consumer-benefiting evolution. The combination of a competitive market for generic drugs, rapid…
Search Posts
News Release
Institute Welcomes Court Ruling on Commercial Free Speech
WASHINGTON, D.C., Dec. 4, 2012 – The Competitive Enterprise Institute applauded the U.S. Second Circuit Court of Appeals decision yesterday, concluding that the…
Op-Eds
Senator Durbin is wrong on energy drinks ban
Several lawmakers have called on the U.S. Food and Drug Administration (FDA) to “do something” to protect the public from the alleged threat of energy…
Hoover Institution
Free Speech for Big Pharma
One of the most important elements of medicine is also among the least well known: the ability of physicians to prescribe approved medicines for purposes…
Blog
Obamacare Will Increase Health Insurance Costs In California, Washington, D.C., And Elsewhere
California officials concede that their state’s Obamacare exchange will hike premiums for policyholders by up to 25 percent. In the District of Columbia, small…
Hoover Institution
A Lack of Government Transparency: The Devil In The Detailing
Cowritten by Jeff Stier. A House Appropriations subcommittee has voted to move forward legislation that would cut $1.3 billion from the Department of Health and…
Orange County Register
A Losing Proposition on Food Labeling
California's initiative process – which allows "propositions" to be placed on the ballot quite easily – can lead to laws that are muddled, intentionally misleading…
Forbes
“Genetically Engineered” In California: A Food Label We Don’t Need
From “food miles” to farmers’ markets, it seems that consumers have never been more interested in the ways their food is grown. That’s one motivation…
Forbes
A Conservative Case for Obamacare?
From Thomas P. Miller’s column in National Review: The response of roughly half of the Senate Republicans was to co-sponsor an alternative bill…
Forbes
Labeling Of Genetically Engineered Foods Is A Losing Proposition
As Joe Six-pack munches Fritos and popcorn during the opening games of the NFL season, does he care what variety of corn was used to…
Boston Herald
Mass. biotech reaps Monsanto deal
From Ira Kantor's article in The Boston Herald: Gregory Conko, senior fellow at the Competitive Enterprise Institute in Washington, D.C., and co-author of…
USA Today
FDA rules won’t do much good
Food-borne illnesses kill as many as 3,000 Americans each year, but consumers should not expect new Food and Drug Administration regulations to help. These rules,…
Real Clear Policy
More FDA Control Does Not Mean More Safety
News outlets are reporting that the U.S. Food and Drug Administration (FDA) has cited an alarmingly high number of alleged safety and regulatory violations by…
Blog
FDA Delay Likely Killed Thousands, Imposed Billions In Costs
The FDA didn’t approve a home test for HIV until 24 years after it first received an application. According to an FDA advisory committee,…
Blog
Dietitian Licensing Board Attempts To Limit Free Speech, Silence Bloggers
Have you ever given someone advice on how to lose weight through dietary changes? Have you ever recommended that certain foods could be consumed or…
Real Clear Policy
The American Revolution Comes to a Pitiful Close
June 2012 – and especially its last week – was ripe with ominous metaphor, all revolving around the Supreme Court’s decision on June 28th to…
Blog
Court’s Obamacare Decision — What Would John Locke Say?
Richard Epstein of the Hoover Institution and the University of Chicago Law School gives the Chief Justice some tough love in “What Was Roberts…
Blog
The Good, the Bad, and the Broccoli
Most people thought that the health care decision would hinge on the Court’s interpretation of the Commerce Clause. That’s why I wrote the first three…
Blog
Obamacare Lives. So, Now What?
Former CEI scholar Tom Miller (now with AEI) has some thoughts on the Obamacare decision in today's…
Blog
CEI Podcast: June 28, 2012: The Obamacare Decision
General Counsel Sam Kazman shares his thoughts on the Supreme Court's health care decision, the Commerce Clause, Congress' taxation power, and more.
Blog
Supreme Court Concocts New “Rational (Tax) Basis” Test in Upholding Health Law
In a move that seems to have surprised many observers, the Supreme Court today upheld nearly all of the Patient Protection and Affordable Care Act…
Blog
Obamacare Upheld, 5-to-4: A Perverse Decision That Undermines Political Accountability
Today, in a really perverse ruling, the Supreme Court upheld Obamacare's individual mandate as a tax in a 5-to-4 decision, even though Obamacare's supporters…
Blog
Quick Thoughts on the Health Care Ruling
The Supreme Court upheld the health care bill, as you've no doubt heard by now. Over at the Daily Caller, I offer a few quick…
Daily Caller
Three Quick Thoughts on the Health Care Ruling
The Supreme Court has upheld the health care law’s insurance mandate, to the surprise of many. This surprise sparked a few quick thoughts about the…
News Release
Supreme Court Concocts “Rational Tax Test” in Health Ruling
Washington, D.C., June 28, 2012 – Statement by CEI Senior Fellow Gregory Conko: In enacting the health care individual purchase mandate, Congress…
Real Clear Markets
Global Governments’ Growing Embrace of Big Pharma
As the pharmaceutical industry wrestles with multiple existential threats-from price controls to patent cliffs to dwindling pipelines-calls are growing for deeper government-industry cooperation. As private…
Bio-IT World
Does Thimerosal Prevent Autism? Epidemiology Gone Wild
Human beings are born pattern recognizers, part of the repertoire of survival skills that separates us from the beasts. We are programmed to spot relationships…
Blog
Supreme Court Issues 5-to-4 Rulings, But Not On Obamacare
The Supreme Court announced four decisions today, three of them decided by slender 5-to-4 margins, but not the long-awaited ruling about the constitutionality of…
Bio-IT World
Should We Label Genetically-Modified Food?
Green State TV is back for 2011 with an interview on biotechnology and agriculture with Greg Conko…
Washington Times
High Price of Cheap Drug Imports
With the Senate set to vote on one of the few “must-pass” bills of the year, pharmaceutical industry critics are plotting ways to add poison…
Blog
More First Amendment Violations from Obamacare, Thanks to HHS
Obamacare will drive up costs for most patients and insurance policyholders. Yet "health-insurance companies must tell customers who get a premium rebate…
Washington Times
ObamaCare’s Killer Device Tax
Much of the political conversation in Washington these days concerns innovation, job creation and competitiveness. But talk is cheap, and elected officials must enact policies…
Washington Times
The Off-Label Drug War of Words
If knowledge is power and ignorance is bliss, what is power used to prevent knowledge from eliminating ignorance? I don’t know; ask the FDA (U.S.
Heartlander
Critical Drug Shortages Reaching Crisis
From Ashley Bateman's article on Heartland.org: But according to Greg Conko, a senior fellow at the Competitive Enterprise Institute, more mandates on companies…
Medical Progress Today
Mayo v. Prometheus and Diagnostic Patents: What Does the Supreme Court Decision Really Mean?
I finally had a chance to read the Supreme Court's recent decision in the Mayo v. Prometheus Labs case, which invalided two patents claiming methods…
Medical Progress Today
The Orphan Drug Conundrum
It’s not a crisis. Yet. Bigger health care issues loom. Right now. There are still fortunes to be made. While it lasts. But one could…
Blog
Economic “Recovery” Is Slow and Weak Due to Obama Administration Policies
Typically, after the economy suffers an unusually severe recession, it bounces back in an unusually rapid recovery -- what some economists and others refer to…
Blog
Obamacare: Constitutionality Argument Misses the Point Entirely
Conservatives are ebullient over the unexpected hostility and skepticism the government's lawyers faced from the Supreme Court Justices over the three days of hearings on…
Blog
No to Broccoli Mandate, Yes to Health Insurance Mandate?
Over at the Daily Caller, I go over some possible explanations for the different results and conclude:…
Blog
Obamacare Harms State Finances, Imposes Unfunded Mandates, Drives Up State Budget Deficits; Even Democrats Criticize Provisions
While public attention has focused on Obamacare's unconstitutional "individual mandate," challenged yesterday in oral arguments at the Supreme Court, other parts of the health…
Medical Progress Today
Stearns’ Healthcare Record Questioned
From Bill Thompson’s article in The Gainesville Sun: Such remarks echo a common refrain among conservative critics of President Barack…
Daily Caller
No to Broccoli Mandate, Yes to Health Insurance Mandate?
The results of a Reason-Rupe poll that was released on Monday are more interesting than the pollsters may have intended. Two of the questions they…
Blog
Supreme Court Begins Hearing Challenges to Unconstitutional Obamacare Provisions
At CNN, George Mason University law professor Ilya Somin explains why Obamacare's requirement that individuals buy health insurance is beyond Congress's power…
Daily Caller
HHS Deems Rate Hikes Excessive in Nine States
From Michael P. Tremoglie’s article on Legal Newsline: But the issue of the government telling insurers how much premiums should be is a…
Blog
Obamacare Costs More Than Twice As Much As Obama Claimed; Stimulus Creates Debt, Not Jobs
As Daniel Foster notes, “When it was being debated, Democrats told you ACA [Obamacare] would cost $940 billion over ten years . . .
Blog
Obamacare: Anyone Have a Plan B?
In just a few week the Supreme Court will hear oral arguments regarding the legal challenges to the administration's controversial health-care overhaul, especially the constitutionality of the so-called "individual mandate" that requires every American to purchase government-approved insurance. The…
Daily Caller
The Anti-GM Food Circus Rolls Through Connecticut
From the American Council on Science and Health’s “Health Facts and Fears”: But as Gregory Conko, a senior fellow at the Competitive Enterprise…
Blog
Taxmageddon Comes Just After the Election
On December 31, shortly after the November election, tax rates will rise across the board in what congressional aides call "Taxmageddon," notes The Washington Post. Not…
Daily Caller
Shoppers already have a choice regarding biotech foods
Consumers increasingly base food purchasing decisions on individual preferences about product content. For many, this means a focus on nutrition or fat. Others care more…
Blog
CEI Podcast for February 16, 2012: Washington’s Prescription Drug Shortage
Patients are suffering from a nationwide shortage of more than 260 different prescription drugs, many of them for different types of cancer. Senior Fellow Greg…
Blog
Congress Seems Intent on Making Drug Shortages Worse
Now that the problem of prescription drug shortages has begun to affect children, members of Congress want to be seen as…
Daily Caller
How is the FDA Really Doing?
I read with interest—and mounting skepticism—Patricia Dimond’s Insight & Intelligence™ piece about FDA, “FDA New Drug Approvals in 2011 Outpace…
Blog
Misconceptions about the Obama Administration’s Contraception Mandate for Religious Employers
There are a number of misconceptions about the Obama administration’s recent rule requiring employers’ health insurance policies (including those of religious schools and hospitals) to…
Blog
Government Thwarts Cancer Cures and Production of Life-Saving Drugs
The federal government thwarted a promising cancer treatment. The Food and Drug Administration (FDA) put Dr. Stanislaw Burzynski on trial twice, saying “it did…
Daily Caller
Vincent Vernuccio explains the union’s latest method of recruitment.
Vincent Vernuccio explains the union's the latest method of forcing home healthcare and daycare workers into their unions…
Daily Caller
Seeking Solution to “Incredibly Complex” Issue of Drug Shortages
Blog
CEI Podcast for February 2, 2012: The FDA’s Latest Power Grab
Fellow in Consumer Policy Studies Michelle Minton breaks down the FDA's behind-the-scenes push to regulate dietary supplements nearly as strictly as prescription drugs.
Blog
Justice Kagan Should Recuse Herself from Obamacare Case
Only in Bizarro World can you claim someone is your attorney -- and thus shielded by attorney work-product privilege -- and then insist in the…
Blog
Michigan SEIU Scam the Product of Government Collective Bargaining
Proponents of government collective bargaining view it as a fundamental human right. The shameful actions of SEIU in Michigan, however, undermine this claim. In…
Daily Caller
Can Brand Makers Be Sued for Generic Drug Injuries?
Trib Live
Criminal Competition
Blog
Doctors Grow Disenchanted With Obamacare’s Costs and Burdens; Health Care Law Arbitrarily Discriminates
69% of physicians are “pessimistic about the future of medicine” because of the 2010 healthcare law, notes Dr. Marc Siegel in USA Today. “Just…
Blog
Obama Administration to People Needing Bone Marrow Transplants: Drop Dead
In December, a federal appeals court ruled in Flynn v. Holder that the National Organ Transplant Act of 1984 (NOTA)…
Blog
War on Drugs Keeps Badly Needed, Perfectly Legal Medicine Away from Sick People
Sick people, like those suffering from narcolepsy, are suffering from a manufacturing shortage of Adderall. That shortage was caused by the Drug Enforcement Agency,…
Blog
Price Fixing Causes Greek Medicine Shortage
Greece is rapidly degenerating into third-world status. The UK’s Daily Mail reports: Youngsters are being dumped by their parents who are struggling to make…
Blog
Obamacare Causes Layoffs in Medical Device Industry, Harms Medical Innovation
Ramesh Ponnuru writes about the layoffs and lost jobs resulting from Obamacare's new tax on medical devices at Bloomberg News: A year from…
Blog
Obamacare Stifles Job Creation, Causes Layoffs
At Bloomberg News, Andrew Puzder, CEO of CKE Restaurants, Inc., explains how the 2010 healthcare law is preventing jobs from being created and resulting…
Western FarmPress
Mandatory GMO Food Labeling Implies Risks Where There Are None
This offering comes under the heading of “Wish I had written that.” In a recent issue of Forbes, Henry I. Miller and Gregory Conko wrote …
Blog
Businessmen: Obamacare Stops Them from Hiring
Journalist John Stossel describes how "three successful businessmen came on" his TV show last week "to explain how Obamacare is a reason that unemployment stays…
Western FarmPress
Stoking Fears About ‘No More Tears’
For as long as there have been cosmetics, they’ve been part of the holidays. They’re popular Christmas gifts and part of looking good at big…
Western FarmPress
The FDA vs. Commercial Speech
The ability of physicians to prescribe approved medicines for purposes not sanctioned by the Food and Drug Administration (FDA) is one of the most important…
Blog
FDA Needs to Act on Internet and Social Media Policy
Way back in September 2009, the Food and Drug Administration announced that it would begin using the social media site Twitter to share news and other…
Blog
Over-The-Counter Plan B? What Would Jed Bartlet Do?
Back in March 2009, President Obama issued a memorandum on scientific integrity to the heads of executive branch agencies and departments.
Western FarmPress
Labeling Of Biotech Foods Is Unnecessary And Unconstitutional
This piece was co-written with Henry Miller. Should the government require that labels on cans of marinara sauce contain information about whether the tomatoes in…
Blog
Good News/Bad News on Compensating Bone Marrow Donors
By now, there's been plenty of news highlighting last week's decision by the Ninth Circuit Court of Appeals that the National Organ…
Blog
Reforming Medical Malpractice Law: Interesting Discussion about Damage Limits in Malpractice Cases
Point of Law has an interesting debate over whether medical-malpractice noneconomic damage caps hurt consumers, between Ted Frank and Shirley Svorny. As…
Blog
Legalizing Kidney Sales Would Save Thousands of Lives, Save Taxpayers a Bundle
Kidney sales should be legal, explains kidney donor Alexander Berger in The New York Times. Berger is a research analyst for GiveWell, a nonprofit that…
Comment
Comments of the Competitive Enterprise Institute Regarding Food and Drug Administration and Food Safety Inspection Service Approaches to Reducing Sodium Consumption
The Competitive Enterprise Institute (CEI) appreciates the opportunity to submit these comments regarding the Food and Drug Administration’s and Food Safety Inspection Service’s Approaches to…
News Release
CEI Condemns Agency Plan to Restrict Salt Availability
Washington, D.C., November 29, 2011 – The Competitive Enterprise Institute today urged the Food and Drug Administration and U.S. Department of Agriculture to reject…
Western FarmPress
Obamacare Sequesters Your Flex Account
Attention Joe and Jane Citizen! Concerned about the fiscal future of your country and your family? Then please step away for five minutes from the…
Blog
Obamacare Attacks Your Flex Account — Minimize Damage in 2013 by Doubling Up for 2012
Hey Joe and Jane Citizen, concerned about the future of your country and your family. Please step away for five minutes from the nonstop TV coverage…
Blog
Sen. Hagan Bill Would Expand Accelerated Drug Approval
According to Bloomberg News, North Carolina Democratic Senator Kay Hagan is set to introduce a bill that would create new “progressive”…
Western FarmPress
The 510(k) Program Should Be Saved, Not Scorned
Blog
Supreme Court Grants Review in Case Challenging Obamacare as Unconstitutional
The Supreme Court today granted certiorari in the Obamacare cases brought by 26 states and the National Federation of Independent Business. The court allotted 5…
Blog
Obama Administration Contributes to Life-Threatening Drug Shortages Even as it Decries Them
In a recent column, Michelle Malkin explained how Obamacare price controls, FDA and DEA rules, and Obama administration policies have contributed to shortages of…
Western FarmPress
Economics at Heart of Drug Shortages
Western FarmPress
Competitive Enterprise Institute Pleased With PDUFA Recommendations
Daily Caller
Regulation Is This Halloween’s Goblin
American entrepreneurs and small business owners have good reason to be scared this Halloween. According to a new Gallup poll, small business owners consider…
Blog
Biotechnology’s 29th Anniversary!
Twenty-nine years ago tomorrow, the U.S. Food and Drug Administration approved Eli Lilly’s and Genentech’s Humulin, making it the first ever fully…
Comment
CEI Comments to the Food and Drug Administration Regarding Medical Device Regulation
Full Document Available in PDF The Competitive Enterprise Institute appreciates the opportunity to submit these comments regarding…
Comment
CEI Comments to the Food and Drug Administration Regarding PDUFA Reauthorization
Full Document Available in PDF The Competitive Enterprise Institute appreciates the opportunity to submit these comments regarding…
Blog
Beware of Asking FDA to Change Itself
Every five years, Congress must reauthorize a piece of legislation called the Prescription Drug User Fee Act (PDUFA), which gives the…
Daily Caller
Seeing Double Regulation
When asked to name the most controversial medical issues of the day, few people would pick eye care. However, in the past half-century, eye…
Daily Caller
Weekend Reading: The True Story of Cosmetics
Daily Caller
Do Cosmetics Really Cause Cancer?
Daily Caller
The EWG Provides Food for (No) Thought
Study
The True Story of Cosmetics
Many environmental groups want to rid the world of synthetic chemicals. Now they are at war with your makeup.
Daily Caller
Think Tank: More FDA 510(k) Oversight Needed, Driving Innovation Overseas
Daily Caller
Competitive Enterprise Institute Criticizes the FDA
Daily Caller
Conservative think tank report blasts FDA
Daily Caller


